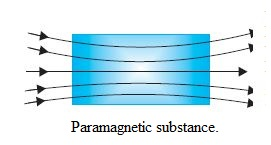
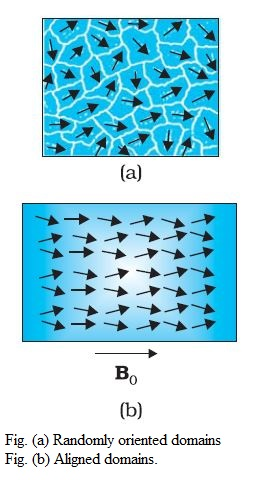
Diamagnetism
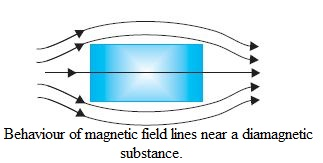
Diamagnetic substances are those which have tendency to move from stronger to the weaker part of the external magnetic field. In other words, unlike the way a magnet attracts metals like iron, it would repel a diamagnetic substance. Figure shows a bar of diamagnetic material placed in an external magnetic field. The field lines are repelled or expelled and the field inside the material is reduced. When placed in a non-uniform magnetic field, the bar will tend to move from high to low field. The simplest explanation for diamagnetism is as follows. Electrons in an atom orbiting around nucleus possess orbital angular momentum. These orbiting electrons are equivalent to current-carrying loop and thus possess orbital magnetic moment. Diamagnetic substances are the ones in which resultant magnetic moment in an atom is zero. When magnetic field is applied, those electrons having orbital magnetic moment in the same direction slow down and those in the opposite direction speed up. This happens due to induced current in accordance with Lenz-s law. Thus, the substance develops a net magnetic moment in direction opposite to that of the applied field and hencerepulsion.
Some diamagnetic materials are bismuth, copper, lead, silicon, nitrogen (at STP), water and sodium chloride. Diamagnetism is present in all the substances. However, the effect is so weak in most cases that it gets shifted by other effects like paramagnetism, ferromagnetism, etc. The most exotic diamagnetic materials are superconductors. These are metals, cooled to very low temperatures which exhibits both perfect conductivity and perfect diamagnetism. Here the field lines are completely expelled! `chi=-1` and `mu_r = 0`. A superconductor repels a magnet and (by Newton-s third law) is repelled by the magnet. The phenomenon of perfect diamagnetism in superconductors is called the Meissner effect, after the name of its discoverer. Superconducting magnets can be gainfully exploited in variety of situations, for example, for running magnetically levitated superfast trains.
Some diamagnetic materials are bismuth, copper, lead, silicon, nitrogen (at STP), water and sodium chloride. Diamagnetism is present in all the substances. However, the effect is so weak in most cases that it gets shifted by other effects like paramagnetism, ferromagnetism, etc. The most exotic diamagnetic materials are superconductors. These are metals, cooled to very low temperatures which exhibits both perfect conductivity and perfect diamagnetism. Here the field lines are completely expelled! `chi=-1` and `mu_r = 0`. A superconductor repels a magnet and (by Newton-s third law) is repelled by the magnet. The phenomenon of perfect diamagnetism in superconductors is called the Meissner effect, after the name of its discoverer. Superconducting magnets can be gainfully exploited in variety of situations, for example, for running magnetically levitated superfast trains.