Davisson-Germer Experiment
The wave nature of electrons was first experimentally verified by C.J. Davisson and L.H. Germer in 1927 and independently by G.P. Thomson, in 1928, who observed diffraction effects with beams of electrons scattered by crystals. Davisson and Thomson shared theNobel Prize in 1937 for their experimental discovery of diffraction of electrons by crystals.
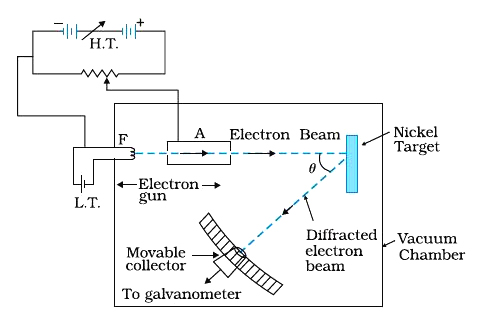
The experimental arrangement used by Davisson and Germer is schematically shown in Fig. It consists of an electron gun which comprises of a tungsten filament F, coated with barium oxide and heated by a low voltage power supply (L.T. or battery). Electrons emitted by the filament are accelerated to a desired velocity by applying suitable potential/voltage from a high voltage power supply (H.T. or battery). They are made to pass through a cylinder with fine holes along its axis, producing a fine collimated beam. The beam is made to fall on the surface of a nickel crystal. The electrons are scattered in all directions by the atoms of the crystal. The intensity of the electron beam, scattered in a given direction, is measured by the electron detector (collector). The detector can be moved on a circular scale and is connected to a sensitive galvanometer, which records the current. The deflection of the galvanometer is proportional to the intensity of the electron beam entering the collector. The apparatus is enclosed in an evacuated chamber. By moving the detector on the circular scale at different positions, the intensity of the scattered electron beam is measured for different values of angle of scattering `theta` which is the angle between the incident and the scattered electron beams. The variation of the intensity (`I`) of the scattered electrons with the angle of scattering `theta` is obtained for different accelerating voltages.
The experiment was performed by varying the accelerating voltage from 44 V to 68 V. It was noticed that a strong peak appeared in the intensity (`I`) of the scattered electron for an accelerating voltage of 54V at a scattering angle `theta=50^0` The appearance of the peak in a particular direction is due to the constructive interference of electrons scattered from different layers of the regularly spaced atoms of the crystals. From the electron diffraction measurements, the wavelength of matter waves was found to be 0.165 nm. The de Broglie wavelength `lamda` associated with electrons, using `lamda=(1.227)/sqrtV`
for V = 54 V is given by
`lamda=h/p=(1.227)/sqrtV`
`lamda=(1.227)/sqrt(54)=0.167`
Thus, there is an excellent agreement between the theoretical value and the experimentally obtained value of de Broglie wavelength. Davisson-Germer experiment thus strikingly confirms the wave nature of electrons and the de Broglie relation. More recently, in 1989, the wave nature of a beam of electrons was experimentally demonstrated in a double-slit experiment, similar to that used for the wave nature of light. Also, in an experiment in 1994, interference fringes were obtained with the beams of iodine molecules, which are about a million times more massive than electrons.
The de Broglie hypothesis has been basic to the development of modern quantum mechanics. It has also led to the field of electron optics. The wave properties of electrons have been utilized in the design of electron microscope which is a great improvement, with higher resolution, over the optical microscope.
The experimental arrangement used by Davisson and Germer is schematically shown in Fig. It consists of an electron gun which comprises of a tungsten filament F, coated with barium oxide and heated by a low voltage power supply (L.T. or battery). Electrons emitted by the filament are accelerated to a desired velocity by applying suitable potential/voltage from a high voltage power supply (H.T. or battery). They are made to pass through a cylinder with fine holes along its axis, producing a fine collimated beam. The beam is made to fall on the surface of a nickel crystal. The electrons are scattered in all directions by the atoms of the crystal. The intensity of the electron beam, scattered in a given direction, is measured by the electron detector (collector). The detector can be moved on a circular scale and is connected to a sensitive galvanometer, which records the current. The deflection of the galvanometer is proportional to the intensity of the electron beam entering the collector. The apparatus is enclosed in an evacuated chamber. By moving the detector on the circular scale at different positions, the intensity of the scattered electron beam is measured for different values of angle of scattering `theta` which is the angle between the incident and the scattered electron beams. The variation of the intensity (`I`) of the scattered electrons with the angle of scattering `theta` is obtained for different accelerating voltages.
The experiment was performed by varying the accelerating voltage from 44 V to 68 V. It was noticed that a strong peak appeared in the intensity (`I`) of the scattered electron for an accelerating voltage of 54V at a scattering angle `theta=50^0` The appearance of the peak in a particular direction is due to the constructive interference of electrons scattered from different layers of the regularly spaced atoms of the crystals. From the electron diffraction measurements, the wavelength of matter waves was found to be 0.165 nm. The de Broglie wavelength `lamda` associated with electrons, using `lamda=(1.227)/sqrtV`
for V = 54 V is given by
`lamda=h/p=(1.227)/sqrtV`
`lamda=(1.227)/sqrt(54)=0.167`
Thus, there is an excellent agreement between the theoretical value and the experimentally obtained value of de Broglie wavelength. Davisson-Germer experiment thus strikingly confirms the wave nature of electrons and the de Broglie relation. More recently, in 1989, the wave nature of a beam of electrons was experimentally demonstrated in a double-slit experiment, similar to that used for the wave nature of light. Also, in an experiment in 1994, interference fringes were obtained with the beams of iodine molecules, which are about a million times more massive than electrons.
The de Broglie hypothesis has been basic to the development of modern quantum mechanics. It has also led to the field of electron optics. The wave properties of electrons have been utilized in the design of electron microscope which is a great improvement, with higher resolution, over the optical microscope.