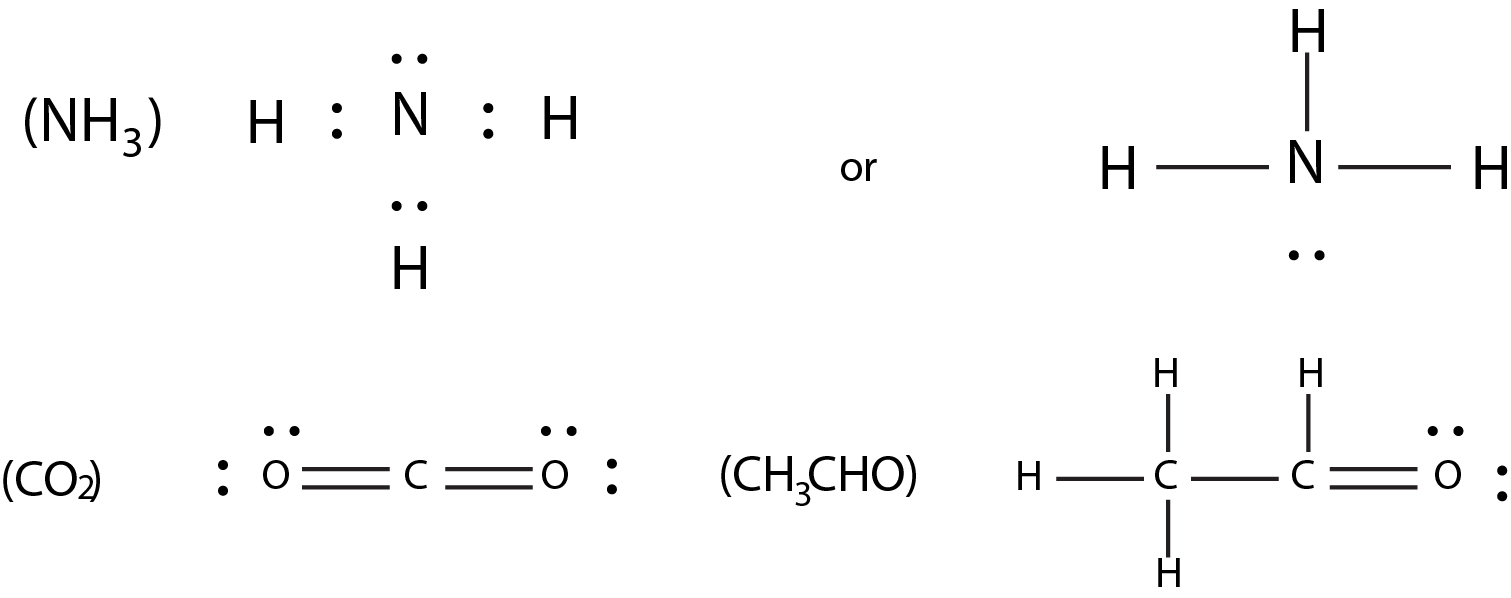
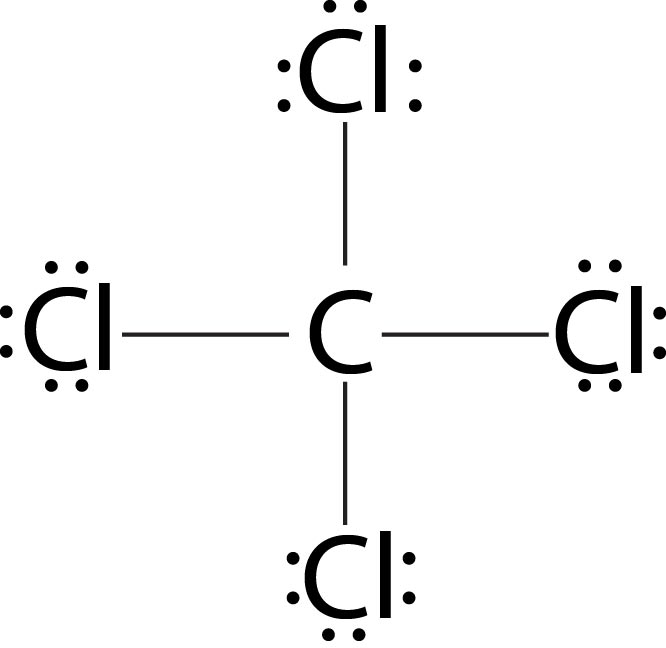
To draw the lewis stryctyres of polyatomic species follow the given sequence
(i) First calculate `n_1`.
`n_1 =` Sum of valence electron of all the atoms of the species `pm` net charge on the species.
For a negatively charged species, electrons are added while for positively charged species, the electrons are subtracted. For an uninegatively charged species, add `1` to the sum of valence electrons and for a dinegatively charged species, add
`2` and so on.
(ii) Then calculate `n_2`.
`n_2 =` ( `8xx`number of atoms other than `H`) `+` (`2xx`number of `H` atoms)
(iii) Subtract `n_1` from `n_2`, which gives `n_3`.
`n_3 = n_2 - n_1 =` number of electrons shared between atoms = number of bonding electrons.
`n_3 /2 =(n_2-n_1)/2=` number of shared (bonding) electron pairs = number of bonds.
(iv) Subtracting `n_3` from `n_1` gives `n_4`.
`n_4 = n_1 - n_3 =` number of unshared electrons or non-bonding electrons.
`n_4/2 =(n_1-n_3)/2=` number of unshared electron pairs = number of lone pairs.
(v) Identify the central atom. Generally, the central atom is the one, which is least electronegative of all the atoms, when the other atoms do not contain hydrogen. When the other atoms are hydrogen only, then the central atom would be the more electronegative atom. Here, you are required to know a bit of chemistry, physics or mathematics won't help.
(vi) Now around the central atom, place the other atoms and distribute the required number of bonds (as calculated in step (iii))& required number of lone pairs (as calculated in step (iv) ), keeping in mind that every atom gets an octet of electrons except hydrogen.
(vii) Then calculate the formal charge on each atom of the species. Formal charge is the difference between the valence electrons in an isolated atom and the number of electrons assigned to that atom in a Lewis Structure.
Formal charge on an atom = number of valence electrons of the atom - (number of shared electrons of that atom + number of unshared electrons of that atom).
Formal charge on an atom = number of valence electrons of the atom - number of bonds formed by that atom - number of unshared electrons (`2xx` lone pairs) of that atom.
For every electron of an atom that is shared in a bond, the `text(number of bonds formed by the atom)` is one. Therefore if an atom forms only one bond (`A- B`), one electron of the bond is that of `A` and other is that of `B`. So the "number of bonds" of A and B each is one. But if the bond were a co-ordinate bond (`A-> B`), then two electrons of `A` are involved in it. This makes the number of bonds of `A` to be `2` and that of `B` to be zero.
(viii) When two adjacent atoms get opposite formal charges, then charges can be removed by replacing the covalent bond between the atoms by a dative (co-ordinate) bond.This bond will have the arrowhead pointing towards the atom with positive formal charge. It is not mandatory to show the dative bonds unless required to do so.
(ix) The given Lewis structure should account for the factual aspects of the molecule like resonance (delocalization), bond length, `p-d` back bonding etc. Sometimes, there are more than one acceptable Lewis structure for a given species. In such cases, we select the most plausible Lewis structure by using formal charges and the following guidelines:
`ast` For neutral molecules, a Lewis structure in which there are no formal charges is preferable to one in which formal charges are present.
`ast` Lewis structures with large formal charges (`+2`, `+3` and/or `-2`, `-3` and so on) are less plausible than those with small formal charges.
`ast` Among Lewis structures having similar distributions of formal charges, the most plausible structure is the one in which negative formal charges are placed on the more electronegative atoms.
To draw the lewis stryctyres of polyatomic species follow the given sequence
(i) First calculate `n_1`.
`n_1 =` Sum of valence electron of all the atoms of the species `pm` net charge on the species.
For a negatively charged species, electrons are added while for positively charged species, the electrons are subtracted. For an uninegatively charged species, add `1` to the sum of valence electrons and for a dinegatively charged species, add
`2` and so on.
(ii) Then calculate `n_2`.
`n_2 =` ( `8xx`number of atoms other than `H`) `+` (`2xx`number of `H` atoms)
(iii) Subtract `n_1` from `n_2`, which gives `n_3`.
`n_3 = n_2 - n_1 =` number of electrons shared between atoms = number of bonding electrons.
`n_3 /2 =(n_2-n_1)/2=` number of shared (bonding) electron pairs = number of bonds.
(iv) Subtracting `n_3` from `n_1` gives `n_4`.
`n_4 = n_1 - n_3 =` number of unshared electrons or non-bonding electrons.
`n_4/2 =(n_1-n_3)/2=` number of unshared electron pairs = number of lone pairs.
(v) Identify the central atom. Generally, the central atom is the one, which is least electronegative of all the atoms, when the other atoms do not contain hydrogen. When the other atoms are hydrogen only, then the central atom would be the more electronegative atom. Here, you are required to know a bit of chemistry, physics or mathematics won't help.
(vi) Now around the central atom, place the other atoms and distribute the required number of bonds (as calculated in step (iii))& required number of lone pairs (as calculated in step (iv) ), keeping in mind that every atom gets an octet of electrons except hydrogen.
(vii) Then calculate the formal charge on each atom of the species. Formal charge is the difference between the valence electrons in an isolated atom and the number of electrons assigned to that atom in a Lewis Structure.
Formal charge on an atom = number of valence electrons of the atom - (number of shared electrons of that atom + number of unshared electrons of that atom).
Formal charge on an atom = number of valence electrons of the atom - number of bonds formed by that atom - number of unshared electrons (`2xx` lone pairs) of that atom.
For every electron of an atom that is shared in a bond, the `text(number of bonds formed by the atom)` is one. Therefore if an atom forms only one bond (`A- B`), one electron of the bond is that of `A` and other is that of `B`. So the "number of bonds" of A and B each is one. But if the bond were a co-ordinate bond (`A-> B`), then two electrons of `A` are involved in it. This makes the number of bonds of `A` to be `2` and that of `B` to be zero.
(viii) When two adjacent atoms get opposite formal charges, then charges can be removed by replacing the covalent bond between the atoms by a dative (co-ordinate) bond.This bond will have the arrowhead pointing towards the atom with positive formal charge. It is not mandatory to show the dative bonds unless required to do so.
(ix) The given Lewis structure should account for the factual aspects of the molecule like resonance (delocalization), bond length, `p-d` back bonding etc. Sometimes, there are more than one acceptable Lewis structure for a given species. In such cases, we select the most plausible Lewis structure by using formal charges and the following guidelines:
`ast` For neutral molecules, a Lewis structure in which there are no formal charges is preferable to one in which formal charges are present.
`ast` Lewis structures with large formal charges (`+2`, `+3` and/or `-2`, `-3` and so on) are less plausible than those with small formal charges.
`ast` Among Lewis structures having similar distributions of formal charges, the most plausible structure is the one in which negative formal charges are placed on the more electronegative atoms.