Different Forms of Hydrogen :
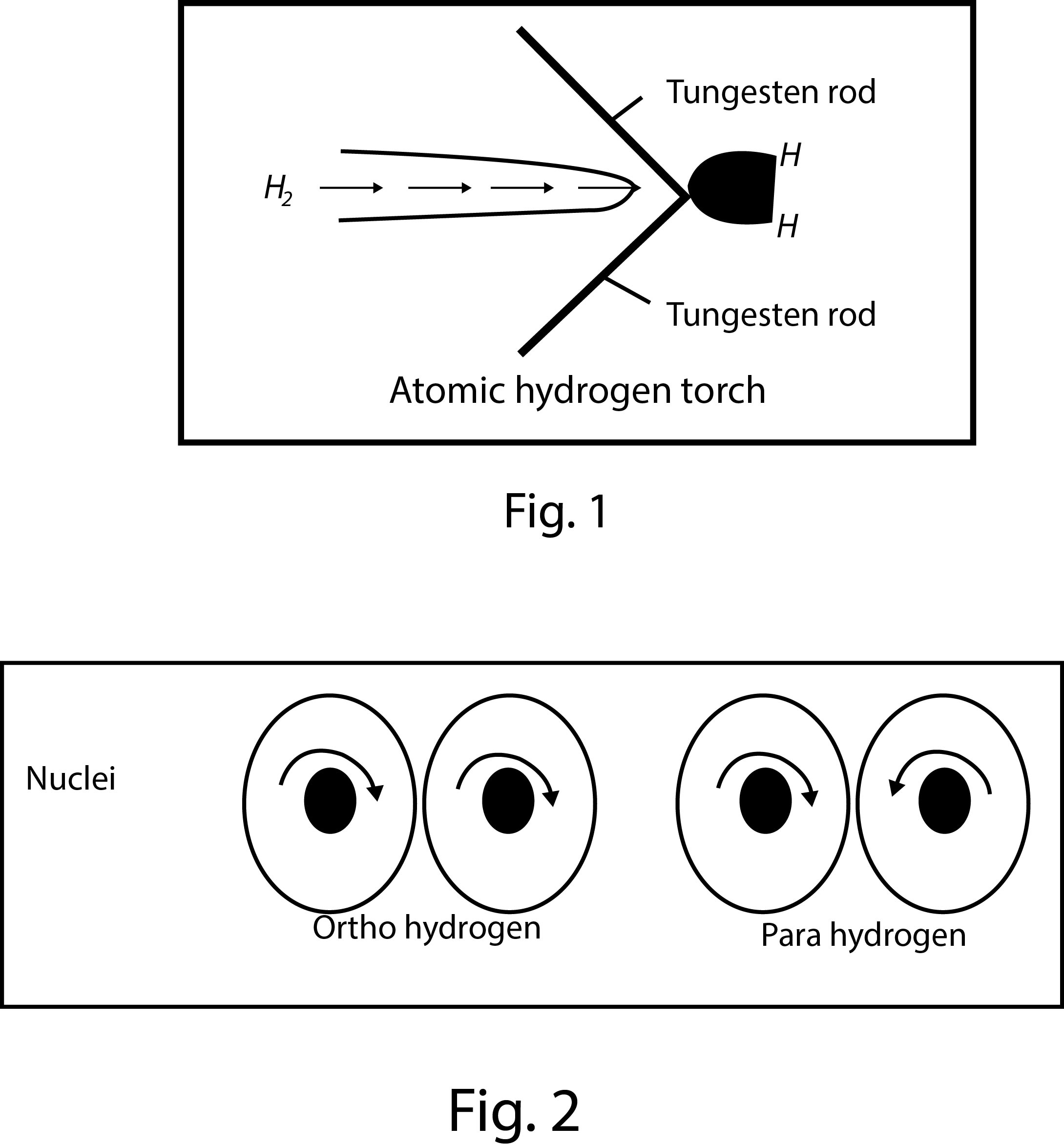
(a) `text(Atomic hydrogen)` : It is obtained by the dissociation of hydrogen molecules. The atomic hydrogen is stable only for a fraction of a second and is extremely reactive. It is obtained by passing dihydrogen gas at atmospheric pressure through an electric arc struck between two tungsten rods.
The electric arc maintains a temperature around `4000-4500^o C`. As the molecules of dihydrogen gas pass through the electric arc, these absorb energy and get dissociated into atoms as
`H_2 (g) undersettext(arc) oversettext(Electric) (->) 2H(g)`; `Delta H = 435 .90 kJ mol^-1`
This arrangement is also called atomic hydrogen torch. See fig.1.
(b) `text(Nascent hydrogen)` : The hydrogen gas prepared in the reaction mixture in contact with the substance with which it has to react, is called nascent hydrogen. It is also called newly born hydrogen. It is more reactive than ordinary hydrogen. For example, if ordinary hydrogen is passed through acidified `KMnO_4` , (pink in colour), its colour is not discharged. On the other hand, if zinc pieces are added to the same solution, bubbles of hydrogen rise through the solution and the colour is discharged due to the reduction on `KMnO_4` by nascent hydrogen.
`KMnO_4 + undersettext(molecular) (H_2) + H_2SO_4 -> text(No reaction)`;
`Zn + H_2SO_4 -> ZnSO_4 + undersettext(Nascent hydrogen) (2[H] xx5)`
`2KMO_4 + 3H_2SO_4 + 10H -> K_2SO_4 + 2MnSO_4 + 8H_2O`
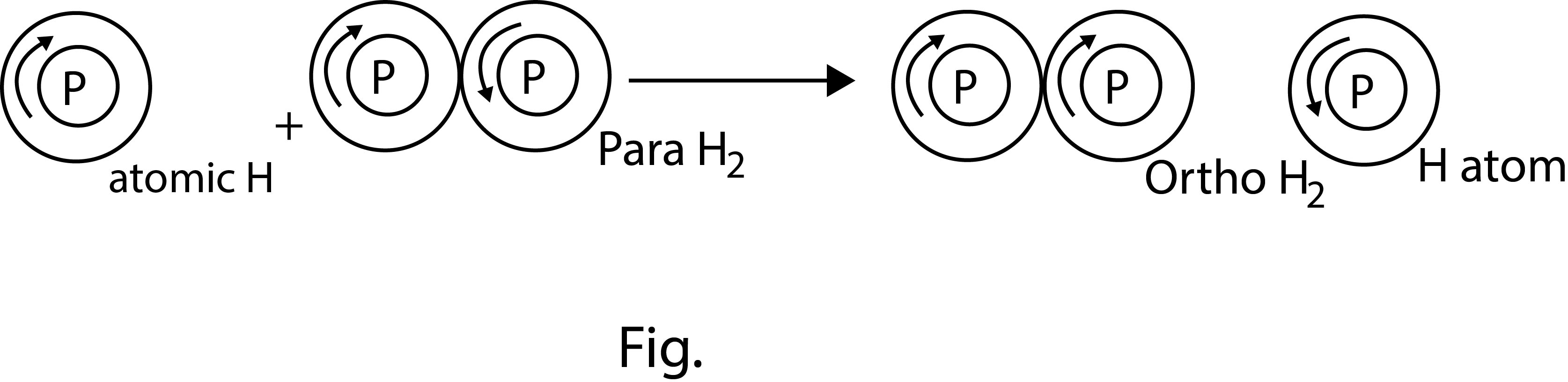
(c) `text(Ortho and para hydrogen)` : A molecule of dihydrogen contains two atoms. The nuclei of both the atoms in each molecule of dihydrogen are spinning(See fig.2). Depending upon the direction of the spin of the nuclei, the hydrogen is of two types-
(i) Molecules of hydrogen in which the spins of both the nuclei are in the same directions, called ortho hydrogen.
(ii) Molecules of hydrogen in which the spins of both the nuclei are in the opposite directions, called para hydrogen.
Ordinary dihydrogen is an equilibrium mixture of ortho and para hydrogen. `text(Ortho hydrogen) ⇋ text(Para hydrogen)`. The amount of ortho and para hydrogen varies with temperature as:
(A) At `0^o` `K`, hydrogen contains mainly para hydrogen which is more stable.
(B) At the temperature of liquefaction of air, the ratio of ortho and para hydrogen is `1:1`.
(C) At the room temperature, the ratio of ortho to para hydrogen is `3:1`.
(D) Even at very high temperatures, the ratio of ortho to para hydrogen can never be more than `3:1`.
Thus, it has been possible to get pure para hydrogen by cooling ordinary hydrogen gas to a very low temperature (close to `20` `K`) but it is never possible to get a sample of hydrogen containing more than 75% of ortho hydrogen. i.e., Pure ortho hydrogen can not be obtained.
The electric arc maintains a temperature around `4000-4500^o C`. As the molecules of dihydrogen gas pass through the electric arc, these absorb energy and get dissociated into atoms as
`H_2 (g) undersettext(arc) oversettext(Electric) (->) 2H(g)`; `Delta H = 435 .90 kJ mol^-1`
This arrangement is also called atomic hydrogen torch. See fig.1.
(b) `text(Nascent hydrogen)` : The hydrogen gas prepared in the reaction mixture in contact with the substance with which it has to react, is called nascent hydrogen. It is also called newly born hydrogen. It is more reactive than ordinary hydrogen. For example, if ordinary hydrogen is passed through acidified `KMnO_4` , (pink in colour), its colour is not discharged. On the other hand, if zinc pieces are added to the same solution, bubbles of hydrogen rise through the solution and the colour is discharged due to the reduction on `KMnO_4` by nascent hydrogen.
`KMnO_4 + undersettext(molecular) (H_2) + H_2SO_4 -> text(No reaction)`;
`Zn + H_2SO_4 -> ZnSO_4 + undersettext(Nascent hydrogen) (2[H] xx5)`
`2KMO_4 + 3H_2SO_4 + 10H -> K_2SO_4 + 2MnSO_4 + 8H_2O`
(c) `text(Ortho and para hydrogen)` : A molecule of dihydrogen contains two atoms. The nuclei of both the atoms in each molecule of dihydrogen are spinning(See fig.2). Depending upon the direction of the spin of the nuclei, the hydrogen is of two types-
(i) Molecules of hydrogen in which the spins of both the nuclei are in the same directions, called ortho hydrogen.
(ii) Molecules of hydrogen in which the spins of both the nuclei are in the opposite directions, called para hydrogen.
Ordinary dihydrogen is an equilibrium mixture of ortho and para hydrogen. `text(Ortho hydrogen) ⇋ text(Para hydrogen)`. The amount of ortho and para hydrogen varies with temperature as:
(A) At `0^o` `K`, hydrogen contains mainly para hydrogen which is more stable.
(B) At the temperature of liquefaction of air, the ratio of ortho and para hydrogen is `1:1`.
(C) At the room temperature, the ratio of ortho to para hydrogen is `3:1`.
(D) Even at very high temperatures, the ratio of ortho to para hydrogen can never be more than `3:1`.
Thus, it has been possible to get pure para hydrogen by cooling ordinary hydrogen gas to a very low temperature (close to `20` `K`) but it is never possible to get a sample of hydrogen containing more than 75% of ortho hydrogen. i.e., Pure ortho hydrogen can not be obtained.