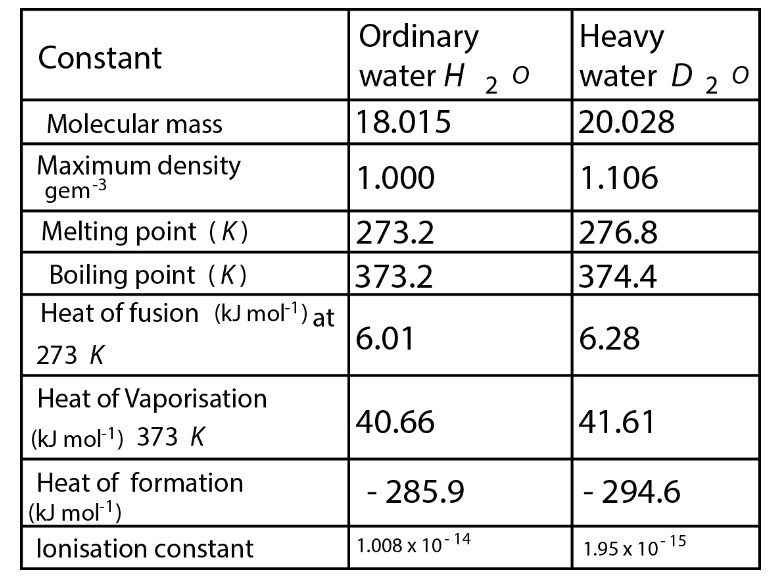
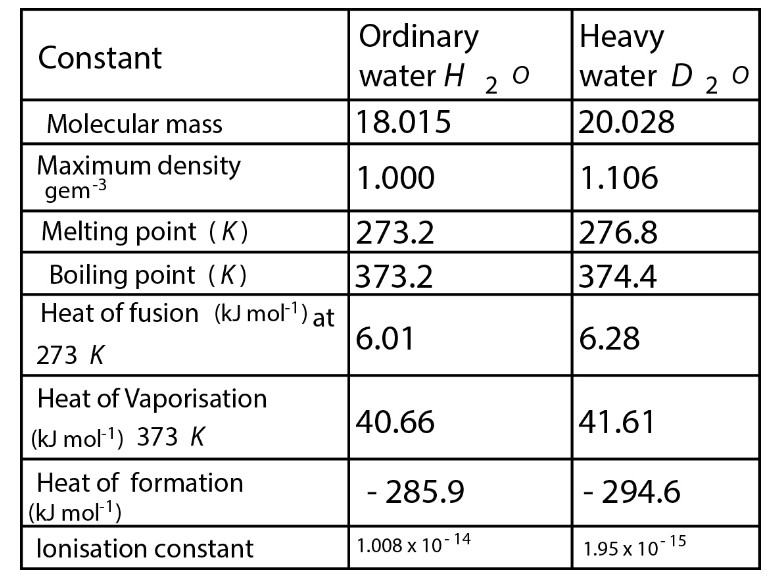
Water & Its Structure :
Water is the oxide of hydrogen. It is an important component of animal and vegetable matter. Water constitutes about `65%` of our body. It is the principal constituent of earth's surface.
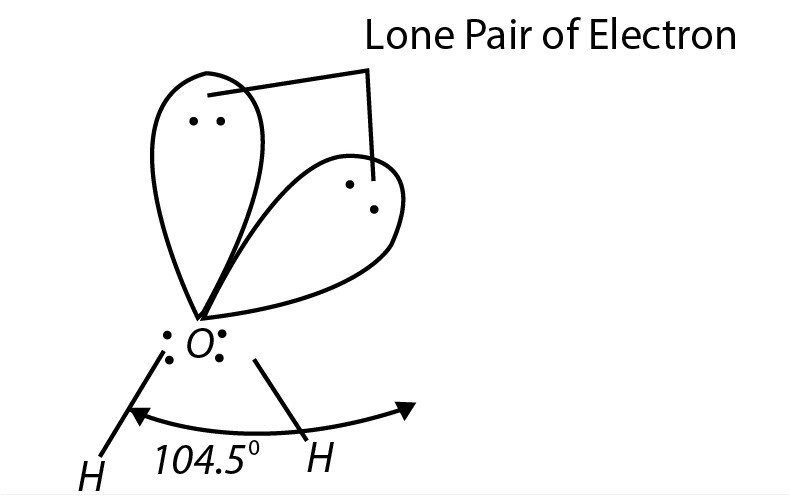
`text(Structure)` : Due to the presence of lone pairs, the geometry of water is distorted and the `H - O- H` bond angle is `104.5^(o)`, which is less than the normal tetrahedral angle (`109.5^(o)`).
The geometry of the molecule is regarded as angular or bent. In water, each `O-H` bond is polar because of the high electronegativity of oxygen (`3.5`) in comparison to that of hydrogen (`2.1`). The resultant dipole moment of water molecule is `1.84D`.
In ice, each oxygen atom is tetrahedrally surrounded by four hydrogen atoms; two by covalent bonds and two by hydrogen bonds. The
resulting structure of ice is open structure having a number of vacant spaces. Therefore, the density of ice is less than that of water and ice floats over water. It may be noted that water has maximum density (`1 g cm^(-3)`) at `4^(o) C`.
`text(Structure)` : Due to the presence of lone pairs, the geometry of water is distorted and the `H - O- H` bond angle is `104.5^(o)`, which is less than the normal tetrahedral angle (`109.5^(o)`).
The geometry of the molecule is regarded as angular or bent. In water, each `O-H` bond is polar because of the high electronegativity of oxygen (`3.5`) in comparison to that of hydrogen (`2.1`). The resultant dipole moment of water molecule is `1.84D`.
In ice, each oxygen atom is tetrahedrally surrounded by four hydrogen atoms; two by covalent bonds and two by hydrogen bonds. The
resulting structure of ice is open structure having a number of vacant spaces. Therefore, the density of ice is less than that of water and ice floats over water. It may be noted that water has maximum density (`1 g cm^(-3)`) at `4^(o) C`.