Lanthanides (4f- block elements) :
Lanthanides are reactive elements so do not found in free state in nature. Most important minerals for lighter Lanthanides are- Monazite, cerites and orthite and for heavier lanthanides - Gadolinite and Xenotime
`text(Electronic configuration)`: The general configuration of lanthanides may be given as `4f^(2-14) 5s^2 5p^6 5d^(0/1)6s^2`. Lanthanide have outer three shells incomplete.
(i) It is to be noted here that filling of `4f` orbitals in the atoms is not regular. A `5d` electron appears in gadolinium (`Z= 64`)with an outer electronic configuration of `4f^7 5d^1 6s^2` (and not `4f^8 6s^2` ). This is because the `4f` and `5d` electrons are at about the same potential energy and that the atoms have a tendency to retain stable half filled configuration.
(ii) On the other hand, the filling of `f`-orbitals is regular in tripositive ions.
(iii) After losing outer electrons, the `f`-orbitals shrink in size and became more stable. `Pm` is the only synthetic radioactive lanthanide.
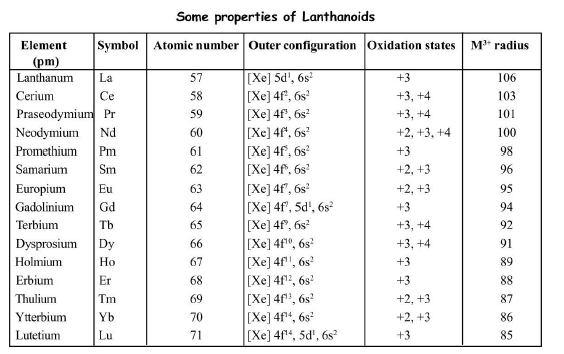
Some properties of Lanthanoids are given in the table.
`text(Electronic configuration)`: The general configuration of lanthanides may be given as `4f^(2-14) 5s^2 5p^6 5d^(0/1)6s^2`. Lanthanide have outer three shells incomplete.
(i) It is to be noted here that filling of `4f` orbitals in the atoms is not regular. A `5d` electron appears in gadolinium (`Z= 64`)with an outer electronic configuration of `4f^7 5d^1 6s^2` (and not `4f^8 6s^2` ). This is because the `4f` and `5d` electrons are at about the same potential energy and that the atoms have a tendency to retain stable half filled configuration.
(ii) On the other hand, the filling of `f`-orbitals is regular in tripositive ions.
(iii) After losing outer electrons, the `f`-orbitals shrink in size and became more stable. `Pm` is the only synthetic radioactive lanthanide.
Some properties of Lanthanoids are given in the table.