Oxidation States :
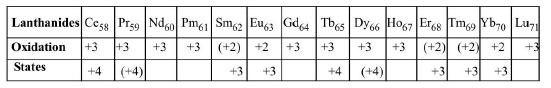
(i) Oxidation states in brackets are unstable states
(ii) The lanthanides contains two `s` electrons in the outermost shell, they are therefore expected to exhibit a characteristic oxidation state of `+2`. But for the lanthanides, the `+3` oxidation is common.
(iii) This corresponds to the use of two outermost electrons (`6s^2`) alongwith one inner electron. The inner electron used is a `5d` electron (in `La`, `Gd` and `Lu`), or one of the `4f` electron if no `5d` electrons present.
(iv) All the lanthanides attains `+3` oxidation state and only cerium, Praseodymium, and terbium exhibit higher oxidation state (`+4`). Oxidation states `+2` and `+4` occur particularly when they lead to
(a) A noble gas configuration e.g. `Ce^(4+) (f^0)`
(b) A half filled 'f' orbital e.g. `Eu^(2+), Tb^(4+), (f^7)`
(c) A completely filled 'f ' orbital e.g. `Yb^(2+) (f^(14)`
Therefore, in higher oxidation state, they act as oxidising while in lower state as reducing agents.
(ii) The lanthanides contains two `s` electrons in the outermost shell, they are therefore expected to exhibit a characteristic oxidation state of `+2`. But for the lanthanides, the `+3` oxidation is common.
(iii) This corresponds to the use of two outermost electrons (`6s^2`) alongwith one inner electron. The inner electron used is a `5d` electron (in `La`, `Gd` and `Lu`), or one of the `4f` electron if no `5d` electrons present.
(iv) All the lanthanides attains `+3` oxidation state and only cerium, Praseodymium, and terbium exhibit higher oxidation state (`+4`). Oxidation states `+2` and `+4` occur particularly when they lead to
(a) A noble gas configuration e.g. `Ce^(4+) (f^0)`
(b) A half filled 'f' orbital e.g. `Eu^(2+), Tb^(4+), (f^7)`
(c) A completely filled 'f ' orbital e.g. `Yb^(2+) (f^(14)`
Therefore, in higher oxidation state, they act as oxidising while in lower state as reducing agents.