Dihydrogen as a Fuel :
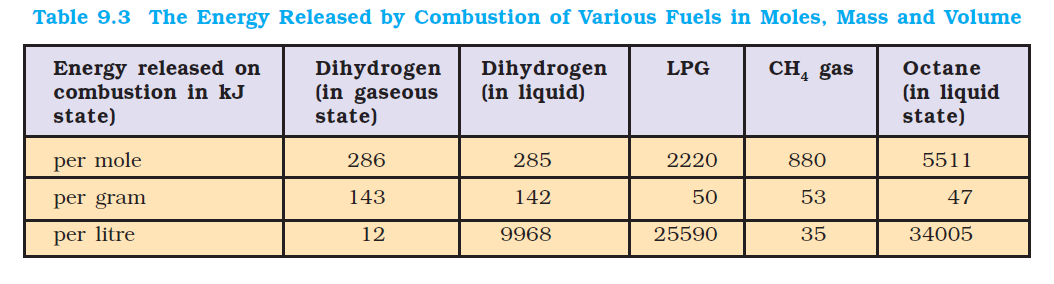
Dihydrogen releases large quantities of heat on combustion. The data on energy released by combustion of fuels like dihydrogen, methane, LPG etc. are compared in terms of the same amounts in mole, mass and volume, are shown in Table 9.3. From this table it is clear that on a mass for mass basis dihydrogen can release more energy than petrol (about three times). Moreover, pollutants in combustion of dihydrogen will be less than petrol. The only pollutants will be the oxides of dinitrogen (due to the presence of dinitrogen as impurity with dihydrogen). This, of course, can be minimised by injecting a small amount of water into the cylinder to lower the temperature so that the reaction between dinitrogen and dioxygen may not take place. However, the mass of the containers in which dihydrogen will be kept must be taken into consideration. A cylinder of compressed dihydrogen weighs about `30` times as much as a tank of petrol containing the same amount of energy. Also, dihydrogen gas is converted into liquid state by cooling to `20K`. This would require expensive insulated tanks. Tanks of metal alloy like `NaNi_5`, `Ti–TiH_2`, `Mg–MgH_2` etc. are in use for storage of dihydrogen in small quantities. These limitations have prompted researchers to search for alternative techniques to use dihydrogen in an efficient way.
In this view `text(Hydrogen Economy)` is an alternative. The basic principle of hydrogen economy is the transportation and storage of energy in the form of liquid or gaseous dihydrogen. Advantage of hydrogen economy is that energy is transmitted in the form of dihydrogen and not as electric power. It is for the first time in the history of India that a pilot project using dihydrogen as fuel was launched in October `2005` for running automobiles. Initially `5%` dihydrogen has been mixed in `text(CNG)` for use in four-wheeler vehicles. The percentage of dihydrogen would be gradually increased to reach the optimum level. Nowadays, it is also used in fuel cells for generation of electric power. It is expected that economically viable and safe sources of dihydrogen will be identified in the years to come, for its usage as a common source of energy.