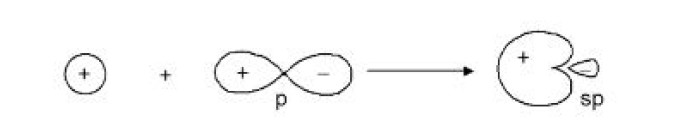
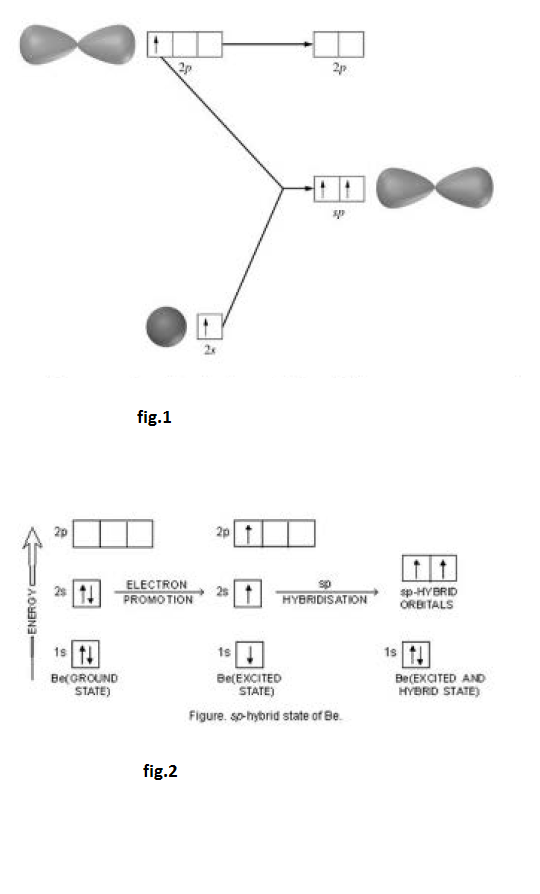
This type of hybridisation involves the mixing of one orbital of `s`-sub-level and two orbitals of `p`-sub-level of the valence shell to form three `sp^2` hybrid orbitals. These `sp^2` hybrid orbitals lie in a plane and are directed towards the corners of equilateral triangle (Fig.1). Each `sp^2` hybrid orbital has one-third `s` -character and two-third `p` -character. `sp^2` hybridisation is also called trigonal hybridisation. The molecules in which central is `sp^2` hybridised and is linked to three other atoms directly have triangular planar shape.
Formation of boron trifluoride `(BF_3)` :
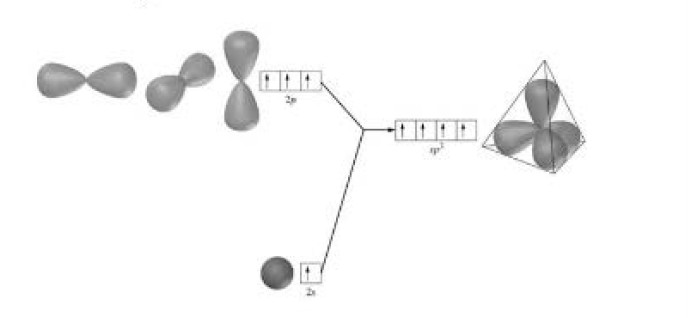
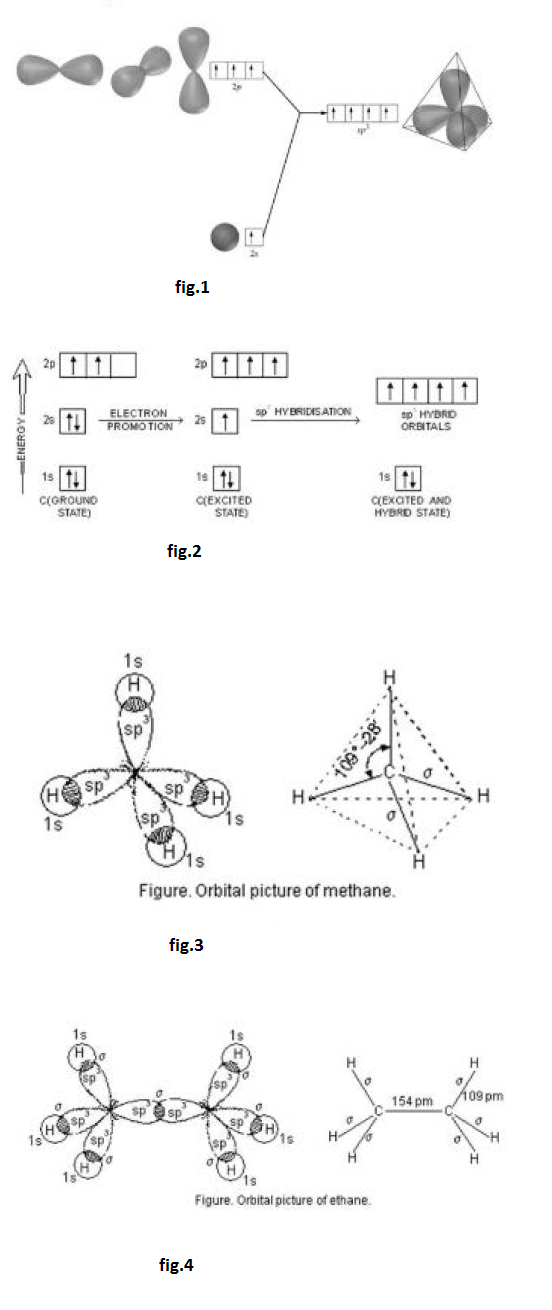
Formation of boron trifluoride (`BF_3`) : Boron (`B`) atom has ground state configuration as `1 s^2 2s^2 2 p^1`. But in the excited state its configuration is `1 s ^2 , 2 s^ 1 , 2 p_x ^1 , 2 p_y ^1`. One `2 s` - orbit of boron intermixes with two `2p-` orbits of excited boron atom to form three `sp^2` hybrid orbital as shown in fig.2.
The `sp^2` hybrid orbitals of boron are directed toward the corners of equilateral triangle and lie in a plane. Each of the `sp^2` hybrid orbitals of boron overlaps axially with half-filled orbital `B-F` sigma bonds a shown in fig.3.
Because of `sp_2` hybridisation of boron, `BF_3` molecule has triangular planar shape.
Formation of ethylene `(C_2H_4)` :
Both the carbon atoms in ethylene assume `sp^2` hybrid state. In acquiring `sp^2` hybrid state, one `2 s` -orbital and two `2 p`-orbitals of excited carbon atom get hybridised to form three `sp^2` hybridised orbitals. However, one orbital of `2 p`-sub-shell of the excited carbon atom does not take part in hybridisation. The promotion of electron and hybridisation in carbon atom is shown in fig.4.
As already indicated, the three `sp^2` hybrid orbitals lie in one plane and are oriented in space at an angle of `120^o` to one another.
The unhybridised `2p` -orbital is perpendicular to the plane of `sp^2` hybrid orbitals as shown in fig.5.
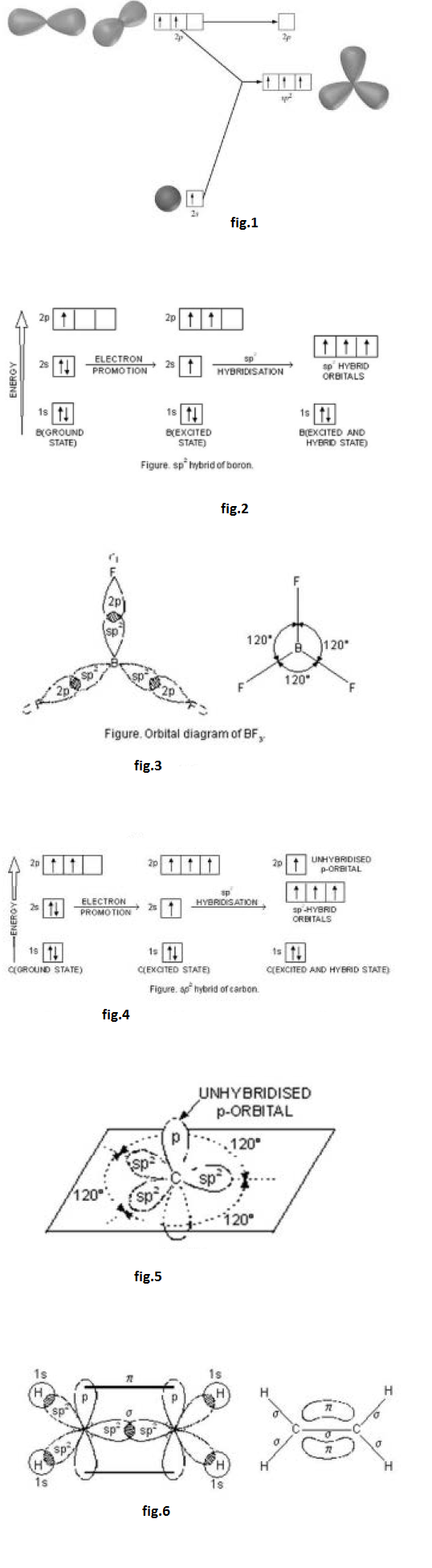
In the formation of ethylene, one of the `sp^2` hybrid orbital of carbon atom overlaps axially with similar orbital of the other carbon atom to form `C-C` sigma bond. The other two `sp^2` hybrid orbitals of each carbon atom are utilised for forming `sp^2`- s sigma bond with two hydrogen atoms.
The unhybridised `p` -orbitals of the two carbon atoms overlap side wise each other to form two `p` clouds distributed above and below the plane of carbon and hydrogen atoms fig.6.
Thus, in ethylene, the six atoms (bonded by sigma bonds) lie in one plane while the p bond is projected perpendicular to the plane of six atoms (two C atoms and four H atoms). In ethylene molecule, the `C = C` bond consists of one `sp^2- sp^2` sigma bond and one p bond. Its bond length is 134 pm. `C - H` bond is `sp^2` - s sigma bond with bond length `108` pm. The `H - C - H ` angle is `117.5^o` while `H- C-C` angle is `121^o` .
This type of hybridisation involves the mixing of one orbital of `s`-sub-level and two orbitals of `p`-sub-level of the valence shell to form three `sp^2` hybrid orbitals. These `sp^2` hybrid orbitals lie in a plane and are directed towards the corners of equilateral triangle (Fig.1). Each `sp^2` hybrid orbital has one-third `s` -character and two-third `p` -character. `sp^2` hybridisation is also called trigonal hybridisation. The molecules in which central is `sp^2` hybridised and is linked to three other atoms directly have triangular planar shape.
Formation of boron trifluoride `(BF_3)` :
Formation of boron trifluoride (`BF_3`) : Boron (`B`) atom has ground state configuration as `1 s^2 2s^2 2 p^1`. But in the excited state its configuration is `1 s ^2 , 2 s^ 1 , 2 p_x ^1 , 2 p_y ^1`. One `2 s` - orbit of boron intermixes with two `2p-` orbits of excited boron atom to form three `sp^2` hybrid orbital as shown in fig.2.
The `sp^2` hybrid orbitals of boron are directed toward the corners of equilateral triangle and lie in a plane. Each of the `sp^2` hybrid orbitals of boron overlaps axially with half-filled orbital `B-F` sigma bonds a shown in fig.3.
Because of `sp_2` hybridisation of boron, `BF_3` molecule has triangular planar shape.
Formation of ethylene `(C_2H_4)` :
Both the carbon atoms in ethylene assume `sp^2` hybrid state. In acquiring `sp^2` hybrid state, one `2 s` -orbital and two `2 p`-orbitals of excited carbon atom get hybridised to form three `sp^2` hybridised orbitals. However, one orbital of `2 p`-sub-shell of the excited carbon atom does not take part in hybridisation. The promotion of electron and hybridisation in carbon atom is shown in fig.4.
As already indicated, the three `sp^2` hybrid orbitals lie in one plane and are oriented in space at an angle of `120^o` to one another.
The unhybridised `2p` -orbital is perpendicular to the plane of `sp^2` hybrid orbitals as shown in fig.5.
In the formation of ethylene, one of the `sp^2` hybrid orbital of carbon atom overlaps axially with similar orbital of the other carbon atom to form `C-C` sigma bond. The other two `sp^2` hybrid orbitals of each carbon atom are utilised for forming `sp^2`- s sigma bond with two hydrogen atoms.
The unhybridised `p` -orbitals of the two carbon atoms overlap side wise each other to form two `p` clouds distributed above and below the plane of carbon and hydrogen atoms fig.6.
Thus, in ethylene, the six atoms (bonded by sigma bonds) lie in one plane while the p bond is projected perpendicular to the plane of six atoms (two C atoms and four H atoms). In ethylene molecule, the `C = C` bond consists of one `sp^2- sp^2` sigma bond and one p bond. Its bond length is 134 pm. `C - H` bond is `sp^2` - s sigma bond with bond length `108` pm. The `H - C - H ` angle is `117.5^o` while `H- C-C` angle is `121^o` .