Formation of Complexes :
By virtue of their small size, comparatively high nuclear or ionic charge and availability of vacant `d`-orbitals of suitable energy, these metals exert strong electrostatic attraction on the ligands. The species formed on interaction of metal and the ligand (or ligands) is known as a complex.
The transition metal ions form complexes because of the following reasons :
(a) Their small cation size.
(b) High effective nuclear charge.
(c) Availability of vacant (`n-1`) `d`-orbitals of appropriate energy.
(d) The structure commonly found in such complex are linear (i.e. co-ordination number, `C.N.=2`), square planer (`C.N. =4`), tetrahedral (`CN = 4`) or octahedral (`CN = 6`).
(e) Cobalt form more complex than any other elements.
`Co^(3+) + 6 NH_3 -> [Co (NH)_6]^(3+)`
`Fe^2 + 6CN^(-) -> [Fe (CN)_6]^(4-)`
`Co^(3+) + 6 H_2O -> [Co(H_2 O)_6]^(3+)`
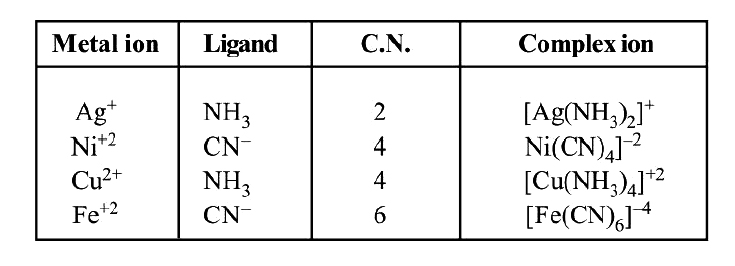
See given table for more examples.
The transition metal ions form complexes because of the following reasons :
(a) Their small cation size.
(b) High effective nuclear charge.
(c) Availability of vacant (`n-1`) `d`-orbitals of appropriate energy.
(d) The structure commonly found in such complex are linear (i.e. co-ordination number, `C.N.=2`), square planer (`C.N. =4`), tetrahedral (`CN = 4`) or octahedral (`CN = 6`).
(e) Cobalt form more complex than any other elements.
`Co^(3+) + 6 NH_3 -> [Co (NH)_6]^(3+)`
`Fe^2 + 6CN^(-) -> [Fe (CN)_6]^(4-)`
`Co^(3+) + 6 H_2O -> [Co(H_2 O)_6]^(3+)`
See given table for more examples.