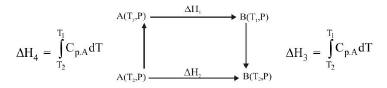
(i) `text(Enthalpy of Formation)`, `Delta_fH` : It is the enthalpy change when one mole of a substance is formed from its elements in their most abundant naturally occurring form (also called reference states).
`H_2(g) + 1/2 O_2(g) -> H_2O(l) ; Delta_f H_(H_2O(l)) = -285.8 kJ` `mol^-1`
`text(Note)` :
(a) By convention, enthalpy of formation `Delta_fH` of an element in reference state is taken as zero.
(b) The enthalpy of formation can be used to determine the enthalpy change of any reaction as
`Delta_r H = underset(i)suma_iDelta_fH_text(products) -underset(i)sum b_iDelta_fH_text(reactamts) `
where `a_i` and `b_i` represent the coefficients of the products and reactants in the balanced chemical equation.
(c) `DeltaH_f` data can be used to compare stability of isomer and allotropes.
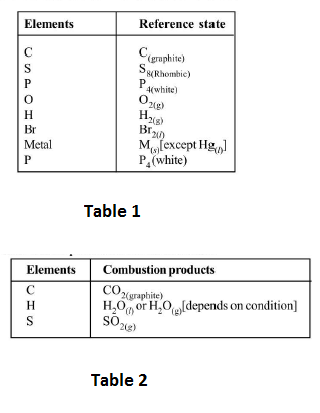
(d) The reference state of commonly used elements are : See Table 1.
(ii) `text(Enthalpy of Combustion)`, `Delta_CH` : lt is the enthalpy change when one mole of the substance undergo complete combustion to give combustion products.
`CH_4(g) + 2O_2(g) -> CO_2(g) + 2H_2O(l)`; `Delta_cH = - 890.8 kJ mol^(-1)` at `298 K`.
The combustion products of the substances are See Table 2.
(iii) `text(Enthalpy of Transition)` : It is the enthalpy change when one mole of one allotropic form changes to another under conditions of constant temperature and pressure. For example
`C(text(graphite)) -> C(text(diamond))`, `Delta_(trs) H = 1.90 kJ mol^(-1)`
(iv) `text(Bond Enthalpies (Bond energies))`, `Delta_text(bond)H` : The bond enthalpy of diatomic molecules like `H_2`, `Cl_2`, `O_2` etc. may be defined as the enthalpy change when one mole of covalent bonds of a gaseous covalent substance is broken to form products in the gas phase, under conditions of constant pressure and temperature. For example
`Cl_2(g) -> 2Cl(g) ; Delta_(Cl-Cl)H = + 242 kJ mol^(-1)`
`O_2(g) -> 2O(g) ; Delta_(O-O)H = + 428 kJ mol^(-1)`
In case of polyatomic molecules, bond dissociation enthalpy is different for different bonds within the same molecule. In such cases, mean bond enthalpy is used. Mean bond enthalpy may be defined as the average enthalpy change to dissociate a particular type of bond in the compounds.
In gas phase reactions, the standard enthalpy of reaction, `Delta_r H^o`, is related with the bond enthalpies of reactants and products as
`Delta_rH^o = sum` bond enthalpies (reactants) `- sum` bond enthalpies (products)
`=sum in` of reactants `- sum in` of products
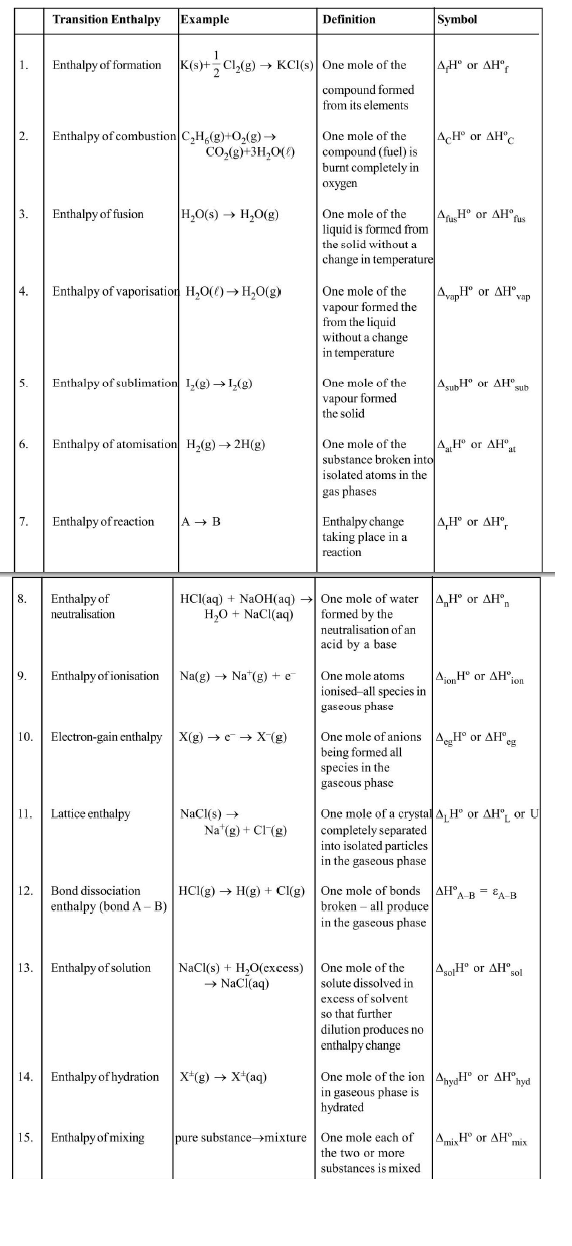
(v) `text(Ionisation Enthalpy)` `(Delta_I H)` : It is the enthalpy change when an electron is removed from an isolated gaseous atom or in its ground state under conditions of constant temperature and pressure.
`X(g) -> X^+ (g) + e^(-)`
(vi) `text(Electron Gain Enthalpy)` `(Delta_(eg)H)` : It is the enthalpy change when an electron is added to a neutral gaseous atom to convert it into a negative ion under conditions of constant temperature and pressure.
`X(g) + e^(-) -> X^(-) (g)`
(vii) `text(Lattice Enthalpy)` `(Delta_(text(lattic))H)` : (The lattice enthalpy of an ionic compound is the enthalpy change which occurs when one mole of an ionic compound dissociates into its ions in gaseous state under conditions of constant temperature and pressure.
`Na^(+) Cl^(-) -> Na^(+)(g) + Cl^(-)(g)`;
`Delta_(text(Lattice))H = + 788 kJ mol^(-1)`
Lattice enthalpy can also be defined for the reverse process. In that case the value of `DeltaH_(LE)` will be negative.
`text(Born- Haber Cycle For NaCl)` : This cycle is based on thermochemical changes taking place in the formation of a lattice. This cycle
can be used to determine lattice energy which cannot be directly measured. It is defined as that energy released when one mole of the ionic compound (lattice) is formed its isolated ions in the gaseous state under standard condition.
`nA^(m+)(g) + mB^(n-)(g) -> A_nB_m(s)`
`Delta H = -U ` (lattice energy)
Formation of `NaCl(s)` lattice involves thus.
`S + I + (epsilon_(Cl-Cl))/2 -E -U =q`
hence, `U` can be calculated.
here, `S = ` enthalpy of sublimation of `Na(s) = Delta H_(text(sublimation))`
`I =` ionisation energy of `Na(g) = DeltaH_(text(ionization))`
`epsilon =` bond energy of `Cl_2`
`U =` lattice energy
`q =` enthalpy of formation of `NaCl(s) = Delta H_(text(formation))`
If lattice is `MgX_2(s)` then
`S + (I_1 + I_2) + epsilon - 2E - U = q`
where, `(I_1 + I_2) =` total ionisation energy to form `Mg^(2+)(g)`.
(viii) `text(Enthalpy of Atomisation)`, `Delta_aH` : It is the enthalpy change when one mole of a substance is completely dissociated into atoms in the gaseous state, under constant pressure and temperature condition.
For example
`H_2(g) -> 2H(g)`; `Delta_a H = 435.0 kJ mol^(- 1)`
`CH_4(g) -> C(g) + 4H(g)`; `Delta_aH = 1665 kJ mol(- 1)`
(ix) `text(Enthalpy of Hydration)`, `Delta_(hyd)H` : It is the enthalpy change when one mole of an anhydrous (or partly hydrated) compound combines with the required number of moles of water to form a specific hydrate at the specified temperature and
pressure. For example :
`CuSO_4(s) + 5H_2O(l) -> CuSO_4*5H_2O(s)`; `Delta_(hyd)H = - 78.20 kJ mol^(- 1)`
(x) `text(Enthalpy of Solution)`, `Delta_(sol)H` : It is the enthalpy change when one mole of a substance is dissolved in a specified amount of solvent under conditions of constant temperature and pressure. When large volume of solvent is taken, the enthalpy change is called enthalpy of solution at infinite dilution. For example :
`NaCl(s) -> NaCl(aq)`; `Delta_(sol)H = + 4 kJ mol^(-1)`
or, `NaCl(s) -> Na^(+) (aq) + Cl^(-)(aq)`; `Delta_(sol)H = + 4kJ mol^(- 1)`
(xi) `text(Enthalpy of Neutralisation)`, `Delta_(n e t)H` : It is the enthalpy change when one g-equivalent of an acid and one g-equivalent of a base undergo complete neutralisation in aqueous solution and all the reactants & products are at the same specified temperature and pressure.
`HCl(aq) + NaOH(aq) -> NaCl(aq) + H_2O(l) ; Delta_(n e t)H = - 57.7 kJ eq^(- 1)`
The enthalpy of neutralisation of strong acid and strong base is always constant (`-57.7 kJ eq^(- 1)`), independent from the acid and base taken. However, the magnitude of enthalpy change of neutralisation decreases when any one of the acid or base taken is weak.
The value (`- 57.7 kJ eq^(- 1)`) is the value when acids and bases are taken in their infinitely diluted state. If acids and basis are having some other concentration, then value will differ.
(i) `text(Enthalpy of Formation)`, `Delta_fH` : It is the enthalpy change when one mole of a substance is formed from its elements in their most abundant naturally occurring form (also called reference states).
`H_2(g) + 1/2 O_2(g) -> H_2O(l) ; Delta_f H_(H_2O(l)) = -285.8 kJ` `mol^-1`
`text(Note)` :
(a) By convention, enthalpy of formation `Delta_fH` of an element in reference state is taken as zero.
(b) The enthalpy of formation can be used to determine the enthalpy change of any reaction as
`Delta_r H = underset(i)suma_iDelta_fH_text(products) -underset(i)sum b_iDelta_fH_text(reactamts) `
where `a_i` and `b_i` represent the coefficients of the products and reactants in the balanced chemical equation.
(c) `DeltaH_f` data can be used to compare stability of isomer and allotropes.
(d) The reference state of commonly used elements are : See Table 1.
(ii) `text(Enthalpy of Combustion)`, `Delta_CH` : lt is the enthalpy change when one mole of the substance undergo complete combustion to give combustion products.
`CH_4(g) + 2O_2(g) -> CO_2(g) + 2H_2O(l)`; `Delta_cH = - 890.8 kJ mol^(-1)` at `298 K`.
The combustion products of the substances are See Table 2.
(iii) `text(Enthalpy of Transition)` : It is the enthalpy change when one mole of one allotropic form changes to another under conditions of constant temperature and pressure. For example
`C(text(graphite)) -> C(text(diamond))`, `Delta_(trs) H = 1.90 kJ mol^(-1)`
(iv) `text(Bond Enthalpies (Bond energies))`, `Delta_text(bond)H` : The bond enthalpy of diatomic molecules like `H_2`, `Cl_2`, `O_2` etc. may be defined as the enthalpy change when one mole of covalent bonds of a gaseous covalent substance is broken to form products in the gas phase, under conditions of constant pressure and temperature. For example
`Cl_2(g) -> 2Cl(g) ; Delta_(Cl-Cl)H = + 242 kJ mol^(-1)`
`O_2(g) -> 2O(g) ; Delta_(O-O)H = + 428 kJ mol^(-1)`
In case of polyatomic molecules, bond dissociation enthalpy is different for different bonds within the same molecule. In such cases, mean bond enthalpy is used. Mean bond enthalpy may be defined as the average enthalpy change to dissociate a particular type of bond in the compounds.
In gas phase reactions, the standard enthalpy of reaction, `Delta_r H^o`, is related with the bond enthalpies of reactants and products as
`Delta_rH^o = sum` bond enthalpies (reactants) `- sum` bond enthalpies (products)
`=sum in` of reactants `- sum in` of products
(v) `text(Ionisation Enthalpy)` `(Delta_I H)` : It is the enthalpy change when an electron is removed from an isolated gaseous atom or in its ground state under conditions of constant temperature and pressure.
`X(g) -> X^+ (g) + e^(-)`
(vi) `text(Electron Gain Enthalpy)` `(Delta_(eg)H)` : It is the enthalpy change when an electron is added to a neutral gaseous atom to convert it into a negative ion under conditions of constant temperature and pressure.
`X(g) + e^(-) -> X^(-) (g)`
(vii) `text(Lattice Enthalpy)` `(Delta_(text(lattic))H)` : (The lattice enthalpy of an ionic compound is the enthalpy change which occurs when one mole of an ionic compound dissociates into its ions in gaseous state under conditions of constant temperature and pressure.
`Na^(+) Cl^(-) -> Na^(+)(g) + Cl^(-)(g)`;
`Delta_(text(Lattice))H = + 788 kJ mol^(-1)`
Lattice enthalpy can also be defined for the reverse process. In that case the value of `DeltaH_(LE)` will be negative.
`text(Born- Haber Cycle For NaCl)` : This cycle is based on thermochemical changes taking place in the formation of a lattice. This cycle
can be used to determine lattice energy which cannot be directly measured. It is defined as that energy released when one mole of the ionic compound (lattice) is formed its isolated ions in the gaseous state under standard condition.
`nA^(m+)(g) + mB^(n-)(g) -> A_nB_m(s)`
`Delta H = -U ` (lattice energy)
Formation of `NaCl(s)` lattice involves thus.
`S + I + (epsilon_(Cl-Cl))/2 -E -U =q`
hence, `U` can be calculated.
here, `S = ` enthalpy of sublimation of `Na(s) = Delta H_(text(sublimation))`
`I =` ionisation energy of `Na(g) = DeltaH_(text(ionization))`
`epsilon =` bond energy of `Cl_2`
`U =` lattice energy
`q =` enthalpy of formation of `NaCl(s) = Delta H_(text(formation))`
If lattice is `MgX_2(s)` then
`S + (I_1 + I_2) + epsilon - 2E - U = q`
where, `(I_1 + I_2) =` total ionisation energy to form `Mg^(2+)(g)`.
(viii) `text(Enthalpy of Atomisation)`, `Delta_aH` : It is the enthalpy change when one mole of a substance is completely dissociated into atoms in the gaseous state, under constant pressure and temperature condition.
For example
`H_2(g) -> 2H(g)`; `Delta_a H = 435.0 kJ mol^(- 1)`
`CH_4(g) -> C(g) + 4H(g)`; `Delta_aH = 1665 kJ mol(- 1)`
(ix) `text(Enthalpy of Hydration)`, `Delta_(hyd)H` : It is the enthalpy change when one mole of an anhydrous (or partly hydrated) compound combines with the required number of moles of water to form a specific hydrate at the specified temperature and
pressure. For example :
`CuSO_4(s) + 5H_2O(l) -> CuSO_4*5H_2O(s)`; `Delta_(hyd)H = - 78.20 kJ mol^(- 1)`
(x) `text(Enthalpy of Solution)`, `Delta_(sol)H` : It is the enthalpy change when one mole of a substance is dissolved in a specified amount of solvent under conditions of constant temperature and pressure. When large volume of solvent is taken, the enthalpy change is called enthalpy of solution at infinite dilution. For example :
`NaCl(s) -> NaCl(aq)`; `Delta_(sol)H = + 4 kJ mol^(-1)`
or, `NaCl(s) -> Na^(+) (aq) + Cl^(-)(aq)`; `Delta_(sol)H = + 4kJ mol^(- 1)`
(xi) `text(Enthalpy of Neutralisation)`, `Delta_(n e t)H` : It is the enthalpy change when one g-equivalent of an acid and one g-equivalent of a base undergo complete neutralisation in aqueous solution and all the reactants & products are at the same specified temperature and pressure.
`HCl(aq) + NaOH(aq) -> NaCl(aq) + H_2O(l) ; Delta_(n e t)H = - 57.7 kJ eq^(- 1)`
The enthalpy of neutralisation of strong acid and strong base is always constant (`-57.7 kJ eq^(- 1)`), independent from the acid and base taken. However, the magnitude of enthalpy change of neutralisation decreases when any one of the acid or base taken is weak.
The value (`- 57.7 kJ eq^(- 1)`) is the value when acids and bases are taken in their infinitely diluted state. If acids and basis are having some other concentration, then value will differ.