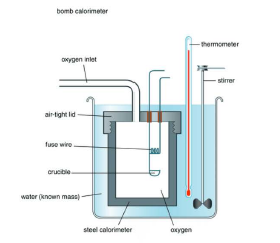
Bomb Calorimeter `DeltaU` Measurement :
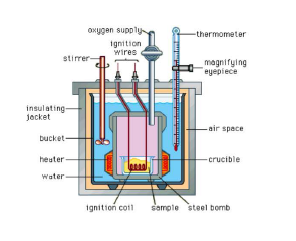
For chemical reactions, heat absorbed at constant volume, is measured in a bomb Calorimeter. In this Calorimeter, a steel vessel (the bomb) is immersed in a water bath. A combustible substance is burnt in pure oxygen supplied in the bomb. Heat evolved during the
reaction is transferred to the water around the bomb and its temperature is monitored. Since the bomb Calorimeter is sealed, its volume does not change, i.e., the energy changes associated with reactions are measured at constant volume.
Since volume does not change, a bomb calorimeter measures the heat evolved under constant volume,
`q_v,`
`q_v = C Delta T,`
where `Delta T` is the temperature increase. The `q_v` so measured is also called the change in internal energy, `= E`.
`= E = q_v = C xx Delta T`
`text(Note)` : More heat is giving of if the reaction is carried out at constant pressure, since the `P-V` work (`1.5 R T`) due to the compression of `1.5` moles of gases in the reactants would contribute to `dH`. lf `1.0` mole water is decomposed by electrolysis at constant pressure, we must supply an amount of energy equivalent to enthalpy change, `dH`, a little more than internal energy, `dE`. More energy must be supplied to perform the `P-V` work to be done by the products (`H_2` and `O_2`).
reaction is transferred to the water around the bomb and its temperature is monitored. Since the bomb Calorimeter is sealed, its volume does not change, i.e., the energy changes associated with reactions are measured at constant volume.
Since volume does not change, a bomb calorimeter measures the heat evolved under constant volume,
`q_v,`
`q_v = C Delta T,`
where `Delta T` is the temperature increase. The `q_v` so measured is also called the change in internal energy, `= E`.
`= E = q_v = C xx Delta T`
`text(Note)` : More heat is giving of if the reaction is carried out at constant pressure, since the `P-V` work (`1.5 R T`) due to the compression of `1.5` moles of gases in the reactants would contribute to `dH`. lf `1.0` mole water is decomposed by electrolysis at constant pressure, we must supply an amount of energy equivalent to enthalpy change, `dH`, a little more than internal energy, `dE`. More energy must be supplied to perform the `P-V` work to be done by the products (`H_2` and `O_2`).