Physical Significance of Entropy :
One can think entropy as a measure of the degree of randomness or disorder in a system. The greater the disorder, in a system, the higher is the entropy.
Prediction of sign of `Delta S` using the concept of Randomness.
(i) With change in temperature at constant `V`
As `T uparrowes S uparrow es => Delta S_text(sys) > 0`
(ii) Change in volume at constant `T`
As `V uparrowes S uparrowes => DeltaS_text(sys) > 0`
(iii) For phase change
`S_text(solid) < S_text(liquid) < S_text(gas)`
(iv) In chemical reaction, entropy change (`Delta S`)
(a) Involving only solids and liquids entropy change will be small
eg. entropy of graphite > diamond (only when we know the structure or any other property).
(b) Involving gases
If `(Delta n)_g > 0 => (DeltaS) > 0`
If `(Delta n)_g < 0 => (Delta S) < 0`
If `(Delta n)_g = 0 => (Delta S) ne 0`
(v) As atomicity `uparrow`es disorder `uparrow`es
`1 mol. quad quad 1mol.`
`S_[NO(g)] < S_[NO_2(g)]`
`S_(CH_4) < S_(C_2H_2)`
(vi) For the molecules having same atomicity, entropy will be more for the substance having more molecular mass.
(vii) In an irreversible process entropy of universe increases but it remains constant in a reversible process.
`Delta S_text(sys) + Delta S_text(surr) =0` for rev. process
`Delta S_text(sys) + Delta S_text(surr) > 0` for irrev. process
`Delta S_text(sys) + Delta S_text(surr) >= 0` ( ln general )
`text[Entropy change in isolated system (isolated system = sys + surr)]`
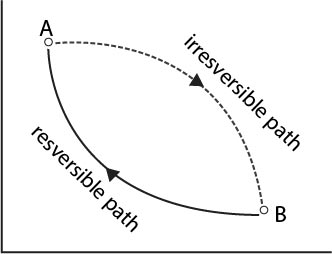
Consider a system taken state `A` to state `B` by an irreversible path and returned to state `A` by a reversible path. Since one of the step is irreversible, according to classius inequality, sum of `q//T` over the cycle must be less than zero(See fig.). Hence
`sum_(A->B)q_text(irr)/T + sum_(B->A)q_text(rev)/T <= 0 => sum_(A->B)q_text(irr)/T <= - sum_(B->A)q_text(rev)/T`
But `sum_(B-> A) q_text(rev)/T = sum_(A-> B) q_text(rev)/T` since the process is reversible
for infinitesimally small change
`[dq_text(rev)]/T_(A-> B) = dS_[text(sys) A-> B]`
`dS_text(system) *[(dq)/T]_(A-> B) > 0`
`Delta S_text(Total isolated sys) > 0`
Entropy calculation in process involving ideal gases.
From First law
`dq =dU + PdV`
`=> [dq_text(rev)]/T = dU/T + (PdV)/T`
But for ideal gas
`(dU)/T = (nC_v dT)/T`
`dS_text(sys) = (nC_v dT)/T + (nR)/V dV`
Integration gives
`Delta S = nC_v ln T_2/T_1 + nR ln (V_2/V_1)`
General Expression, for any process
`Delta S = nC_v ln T_2/T_1 + nR ln V_2/V_1 = nC_p ln T_2/T_1 + nR ln P_1/P_2`
Prediction of sign of `Delta S` using the concept of Randomness.
(i) With change in temperature at constant `V`
As `T uparrowes S uparrow es => Delta S_text(sys) > 0`
(ii) Change in volume at constant `T`
As `V uparrowes S uparrowes => DeltaS_text(sys) > 0`
(iii) For phase change
`S_text(solid) < S_text(liquid) < S_text(gas)`
(iv) In chemical reaction, entropy change (`Delta S`)
(a) Involving only solids and liquids entropy change will be small
eg. entropy of graphite > diamond (only when we know the structure or any other property).
(b) Involving gases
If `(Delta n)_g > 0 => (DeltaS) > 0`
If `(Delta n)_g < 0 => (Delta S) < 0`
If `(Delta n)_g = 0 => (Delta S) ne 0`
(v) As atomicity `uparrow`es disorder `uparrow`es
`1 mol. quad quad 1mol.`
`S_[NO(g)] < S_[NO_2(g)]`
`S_(CH_4) < S_(C_2H_2)`
(vi) For the molecules having same atomicity, entropy will be more for the substance having more molecular mass.
(vii) In an irreversible process entropy of universe increases but it remains constant in a reversible process.
`Delta S_text(sys) + Delta S_text(surr) =0` for rev. process
`Delta S_text(sys) + Delta S_text(surr) > 0` for irrev. process
`Delta S_text(sys) + Delta S_text(surr) >= 0` ( ln general )
`text[Entropy change in isolated system (isolated system = sys + surr)]`
Consider a system taken state `A` to state `B` by an irreversible path and returned to state `A` by a reversible path. Since one of the step is irreversible, according to classius inequality, sum of `q//T` over the cycle must be less than zero(See fig.). Hence
`sum_(A->B)q_text(irr)/T + sum_(B->A)q_text(rev)/T <= 0 => sum_(A->B)q_text(irr)/T <= - sum_(B->A)q_text(rev)/T`
But `sum_(B-> A) q_text(rev)/T = sum_(A-> B) q_text(rev)/T` since the process is reversible
for infinitesimally small change
`[dq_text(rev)]/T_(A-> B) = dS_[text(sys) A-> B]`
`dS_text(system) *[(dq)/T]_(A-> B) > 0`
`Delta S_text(Total isolated sys) > 0`
Entropy calculation in process involving ideal gases.
From First law
`dq =dU + PdV`
`=> [dq_text(rev)]/T = dU/T + (PdV)/T`
But for ideal gas
`(dU)/T = (nC_v dT)/T`
`dS_text(sys) = (nC_v dT)/T + (nR)/V dV`
Integration gives
`Delta S = nC_v ln T_2/T_1 + nR ln (V_2/V_1)`
General Expression, for any process
`Delta S = nC_v ln T_2/T_1 + nR ln V_2/V_1 = nC_p ln T_2/T_1 + nR ln P_1/P_2`