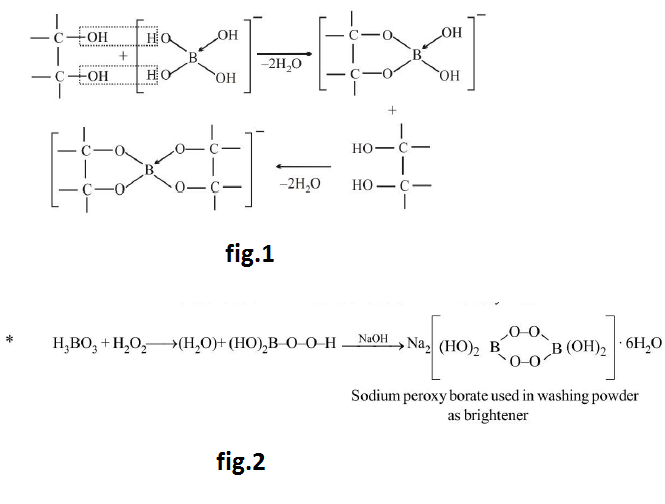
`B_2H_6` (Diborane) :
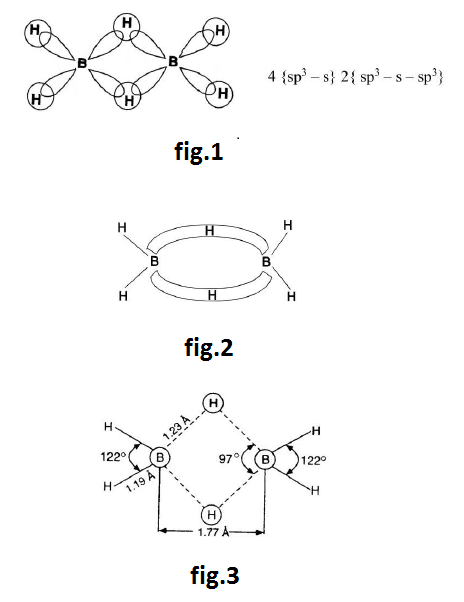
Structure of Diborane : See fig.1.
`B = 1s^2, 2s^2, 2p^1`
`(1s^2 , 2s^1 ,2p_x^1 , 2p_y^1 ,2p_z)/(sp^3 text(Hybridisation))`
(a) `4` Terminal `H`-are bonded by `sigma` bond & remaining `2H` are bridging hydrogens and of these are broken then dimer become monomer.
(b) Boron undergoes `sp^3` hybridisation. `3` of its `sp^3` hybridised orbitals contain one `e^-` each & fourth `sp^3` hybrid orbital is vacant.
(c) `3` of these `sp^3` hybrid orbitals get overlapped by `s` orbitals of `3` hydrogen atoms.
(d) One of the `sp^3` hybrid orbitals which have been overlapped by `s` orbital of hydrogen gets overlapped by vacant `sp^3` hybrid orbital of `2^(nd)` Boron atom and its vice versa.
(e) By this two types of overlapping take place `4(sp^3- s)` overlap bonds & `2 (sp^3 - s - sp^3)` overlap bonds
(f) `H` is held in this bond by forces of attraction from `B` & this bond is called `3` centered two electron bonds. It is also called Banana bonds. Due to repulsion between the two hydrogen nuclei, the delocalised orbitals of bridges are bent away from each other on the middle giving the shape of banana. See fig.2 & fig.3.
`B = 1s^2, 2s^2, 2p^1`
`(1s^2 , 2s^1 ,2p_x^1 , 2p_y^1 ,2p_z)/(sp^3 text(Hybridisation))`
(a) `4` Terminal `H`-are bonded by `sigma` bond & remaining `2H` are bridging hydrogens and of these are broken then dimer become monomer.
(b) Boron undergoes `sp^3` hybridisation. `3` of its `sp^3` hybridised orbitals contain one `e^-` each & fourth `sp^3` hybrid orbital is vacant.
(c) `3` of these `sp^3` hybrid orbitals get overlapped by `s` orbitals of `3` hydrogen atoms.
(d) One of the `sp^3` hybrid orbitals which have been overlapped by `s` orbital of hydrogen gets overlapped by vacant `sp^3` hybrid orbital of `2^(nd)` Boron atom and its vice versa.
(e) By this two types of overlapping take place `4(sp^3- s)` overlap bonds & `2 (sp^3 - s - sp^3)` overlap bonds
(f) `H` is held in this bond by forces of attraction from `B` & this bond is called `3` centered two electron bonds. It is also called Banana bonds. Due to repulsion between the two hydrogen nuclei, the delocalised orbitals of bridges are bent away from each other on the middle giving the shape of banana. See fig.2 & fig.3.