Measurement of Rate of Reaction by Graphs :
(A) `text(Measurement of average rate of reaction)` :
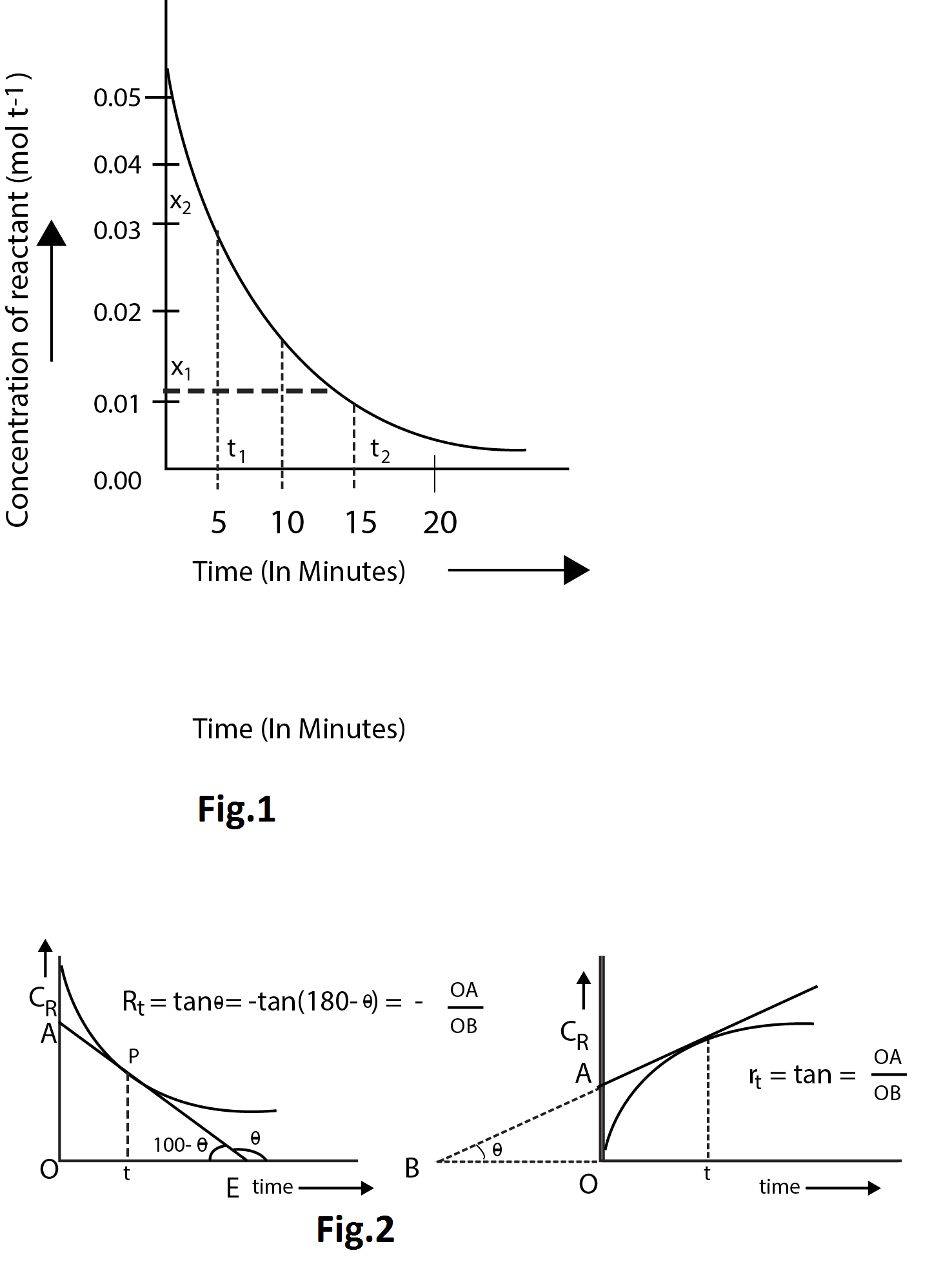
To calculate the average rate of reaction between any two instants of time say `t_1` and `t_2`, the corresponding concentration `x_1` and `x_2` are noted from the graph. Then
Average rate of reaction `= (x_2-x_1)/(t_2-t_1)`
For example, from the fig.1, between the time interval ` 5` to `15` minutes,
Average rate `= (0.03-0.02)/(15-5)=(0.018)/10=0.0018` `mol L^(-1) min^(-1)`
(B) `text(Measurement of Instantaneous rate of reaction)` :
The rate of reaction at any time `t` is determined in the following way,
i) Concentration of any of the reactants or products whichever may be convenient is determined at various time intervals.
ii) Then concentration vs time curve is drawn.
iii) A tangent is drawn at the point `p` of the curve which corresponds to the time `t` at which rate is to be determined.
iv) The slope of the tangent gives the rate of reaction at the required time as shown in fig.2.
(`C_R` and `C_P` denote concentration of reactant and product respectively)
To calculate the average rate of reaction between any two instants of time say `t_1` and `t_2`, the corresponding concentration `x_1` and `x_2` are noted from the graph. Then
Average rate of reaction `= (x_2-x_1)/(t_2-t_1)`
For example, from the fig.1, between the time interval ` 5` to `15` minutes,
Average rate `= (0.03-0.02)/(15-5)=(0.018)/10=0.0018` `mol L^(-1) min^(-1)`
(B) `text(Measurement of Instantaneous rate of reaction)` :
The rate of reaction at any time `t` is determined in the following way,
i) Concentration of any of the reactants or products whichever may be convenient is determined at various time intervals.
ii) Then concentration vs time curve is drawn.
iii) A tangent is drawn at the point `p` of the curve which corresponds to the time `t` at which rate is to be determined.
iv) The slope of the tangent gives the rate of reaction at the required time as shown in fig.2.
(`C_R` and `C_P` denote concentration of reactant and product respectively)