Order of reaction :
The mathematical expression showing the dependence of rate on the concentration(s) of reactant(s) is known as rate-law or rate expression of the reaction and sum of the indices (powers) of the concentration terms appearing in the rate law as observed experimentally is called order of reaction. To understand what is order of reaction, consider the reaction:
`2NO(g)+2H_2(g)-> N_2(g)+2H_2O(g)`
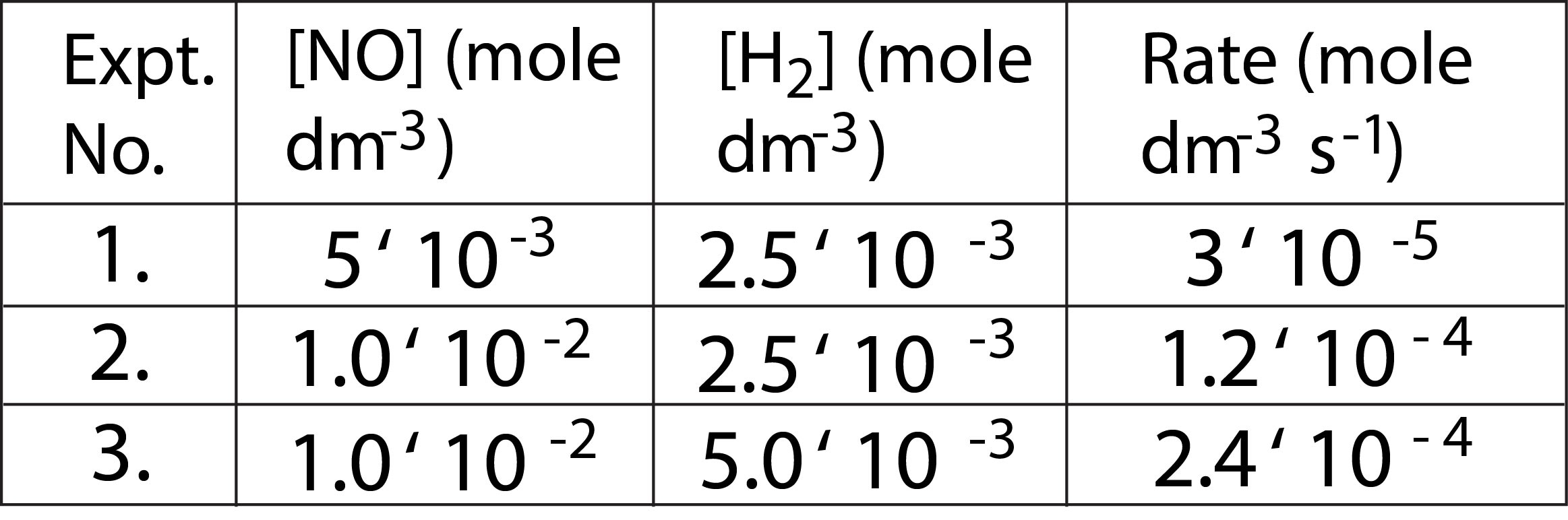
Kinetic experiment carried out at `1100 K` upon this reaction has shown following rate data(See table).
From the Expt. No.1 and 2, it is evident that rate increases `4` fold when concentration of `NO` is doubled keeping the concentration of `H_2` constant i.e.
Rate = `[NO]^2` when `[H _2]` is constant
Again from Expt. No.2 and 3, it is evident that when concentration of `H_2` is doubled keeping the concentration of `NO` constant, the rate is just doubled i.e.
Rate = `[H_2]` when `[NO] ` is constant
From Expt. (1) and Ex pt. (3), the rate increases `8`-fold when concentrations of both `NO` and `H_2` are doubled simultaneously i.e.
Rate = `[NO]^2[H_2]`
This is the rate-law of reaction as observed experimentally. In the rate law, the power of nitric oxide concentration is `2` while that of hydrogen concentration is `1`. So, order of reaction w.r.t. `NO` is `2` and that w.r.t. `H_2` is `1` and overall order is `2 + 1` i.e. `3`. Note that the experimental rate law is not consistent with the stoichiometric coefficient of `H_2` in the chemical equation for the reaction. This fact immediately suggests that the reaction is complicated and it does not occur in single step as written.
`2NO(g)+2H_2(g)-> N_2(g)+2H_2O(g)`
Kinetic experiment carried out at `1100 K` upon this reaction has shown following rate data(See table).
From the Expt. No.1 and 2, it is evident that rate increases `4` fold when concentration of `NO` is doubled keeping the concentration of `H_2` constant i.e.
Rate = `[NO]^2` when `[H _2]` is constant
Again from Expt. No.2 and 3, it is evident that when concentration of `H_2` is doubled keeping the concentration of `NO` constant, the rate is just doubled i.e.
Rate = `[H_2]` when `[NO] ` is constant
From Expt. (1) and Ex pt. (3), the rate increases `8`-fold when concentrations of both `NO` and `H_2` are doubled simultaneously i.e.
Rate = `[NO]^2[H_2]`
This is the rate-law of reaction as observed experimentally. In the rate law, the power of nitric oxide concentration is `2` while that of hydrogen concentration is `1`. So, order of reaction w.r.t. `NO` is `2` and that w.r.t. `H_2` is `1` and overall order is `2 + 1` i.e. `3`. Note that the experimental rate law is not consistent with the stoichiometric coefficient of `H_2` in the chemical equation for the reaction. This fact immediately suggests that the reaction is complicated and it does not occur in single step as written.