Half-time or half-life period of a first order reaction :
The half-time of a reaction is defined as the time required to reduce the concentration of the reactant to half of its initial value. It is denoted by the symbol `t_(1//2)`. Thus,
When `x=a/2,t=t_(1//2)`
Putting these in equation 2 mentioned above, we get
`k=2.303/t_(1//2) log a/(a-a/2)-2.303/(t_1//2) xx 0.30103` `( log 2=0.30103)`
`t_(1//2)=0.693/k`.....(3)
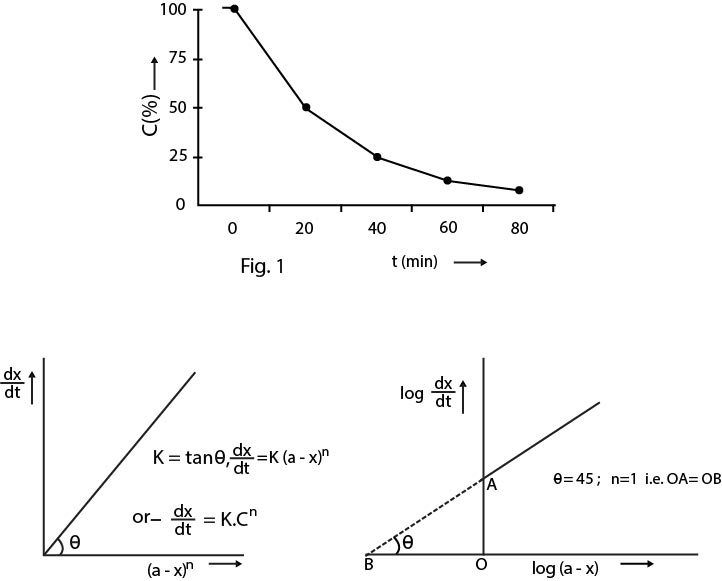
Since `k` is a constant for a given reaction at a given temperature and the expression lacks any concentration term so from equation (3) it is evident that half-time of a `1st` order reaction is a constant independent of initial concentration of reactant . This means if we start with `4` mole `L^(-1)` of a reactant reacting by first-order kinetics and after `20` minute it is reduced to `2` mole `L ^(- 1)`. Its half life will be `20` minute. That is, after `20` minutes from the start of reaction the concentration of the reactant will be `2` mole `L^(-1)`, after `40` minutes from the start of reaction of concentration is `1` mole `L^(-1)`. After `60` minutes from the start of reaction the concentration of the reactant will be reduced to `0.5` mole `L ^(- 1)`. In other words, if during `20` minute `50%` of the reaction completes, then in `40` minute `75%`, in `60` minute `87.5%` of the reaction and on will complete as shown in the fig.1.
Fraction left after `n` half-lives `= (1/2)^n` Concentration left after `n` half lives `a_n =(1/2)^n a_0`
It is also to be noted that equation (3) helps to calculate `t_(1//2)` or `k` with the knowledge of `k` or `t_(1//2)`.
A general expression for `t_(1//2)` is as follows
`t_(1//2) prop 1/(a^(n-1))`
Where `n =` order of reaction.
Graphical Representation for `n^(th)` Order Reaction :
So, from this it is evident that a plot of `(dx)/(dt)` vs `(a -x)^n` will be a straight line passing through the origin and will have its slope equal to `k`, the rate constant of reaction. Thus, for a first order reaction one will get straight line passing through the origin of `(dx)/(dt)` i.e. rate of reaction be plotted against `a - x` as shown in fig.2.
Taking logarithm of the above equation `log= n log (a - x) +log k` This equation tells that a plot of `log (dx)/(dt)` vs `log (a -x)` will be straight line of the slope equal to `n`, order of reaction and intercept equal to `log k`. For first order reaction this slope will be `1` as
shown below. Equation may be rearranged as `log (a - x) = (k/2.303) t + log a`
Thus, a plot of log `(a - x)` vs. `t` will be straight line with slope equal to `-k/2.303` and intercept equal to `log a`, if the reaction is of first order.
When `x=a/2,t=t_(1//2)`
Putting these in equation 2 mentioned above, we get
`k=2.303/t_(1//2) log a/(a-a/2)-2.303/(t_1//2) xx 0.30103` `( log 2=0.30103)`
`t_(1//2)=0.693/k`.....(3)
Since `k` is a constant for a given reaction at a given temperature and the expression lacks any concentration term so from equation (3) it is evident that half-time of a `1st` order reaction is a constant independent of initial concentration of reactant . This means if we start with `4` mole `L^(-1)` of a reactant reacting by first-order kinetics and after `20` minute it is reduced to `2` mole `L ^(- 1)`. Its half life will be `20` minute. That is, after `20` minutes from the start of reaction the concentration of the reactant will be `2` mole `L^(-1)`, after `40` minutes from the start of reaction of concentration is `1` mole `L^(-1)`. After `60` minutes from the start of reaction the concentration of the reactant will be reduced to `0.5` mole `L ^(- 1)`. In other words, if during `20` minute `50%` of the reaction completes, then in `40` minute `75%`, in `60` minute `87.5%` of the reaction and on will complete as shown in the fig.1.
Fraction left after `n` half-lives `= (1/2)^n` Concentration left after `n` half lives `a_n =(1/2)^n a_0`
It is also to be noted that equation (3) helps to calculate `t_(1//2)` or `k` with the knowledge of `k` or `t_(1//2)`.
A general expression for `t_(1//2)` is as follows
`t_(1//2) prop 1/(a^(n-1))`
Where `n =` order of reaction.
Graphical Representation for `n^(th)` Order Reaction :
So, from this it is evident that a plot of `(dx)/(dt)` vs `(a -x)^n` will be a straight line passing through the origin and will have its slope equal to `k`, the rate constant of reaction. Thus, for a first order reaction one will get straight line passing through the origin of `(dx)/(dt)` i.e. rate of reaction be plotted against `a - x` as shown in fig.2.
Taking logarithm of the above equation `log= n log (a - x) +log k` This equation tells that a plot of `log (dx)/(dt)` vs `log (a -x)` will be straight line of the slope equal to `n`, order of reaction and intercept equal to `log k`. For first order reaction this slope will be `1` as
shown below. Equation may be rearranged as `log (a - x) = (k/2.303) t + log a`
Thus, a plot of log `(a - x)` vs. `t` will be straight line with slope equal to `-k/2.303` and intercept equal to `log a`, if the reaction is of first order.