Crystalline Allotropic Forms of Carbon :
Diamond, Graphite and Fullerene
(A) Diamond :
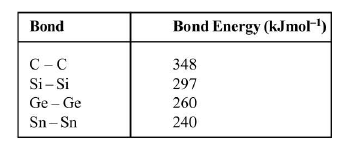
(a) Each carbon is linked to another atom and so very closed packing in structure of Diamond.
(b) Density and hardness is very much greater for diamond because of closed packing in diamond due to `sp^3` hybrid and are tetrahedrally arranged around it.
(c) Diamond has sharp cutting edges that's why it is employed in cutting of glass.
(d) Diamond crystals are non conductor of electricity because of not presence of mobile electron.
(e) `1` carat of diamond = `200` mgm.
(f) Diamond powder if consumed is fatal and causes death in minutes.
(B) Graphite :
(a) In graphite carbon are `sp^2` hybridised and due to this carbon exist as hexagonal layer.
(b) Each carbon is lined with `3` carbons and one carbon will be left and form a two dimensional shed Iike structure.
(c) Distance between two layers is very large so no regular bond is formed between two layers. The layers are attached with weak van der Waal force of attraction.
(d) The carbon have unpaired electron so graphite is a good conductor of current.
(e) `C-C` bond length in Graphite is shorter (`1.42` `A^o`) than that of Diamond (`1.54` `A^o`).
(f) Graphite has high melting point so it is employed in manufacture of crucible.
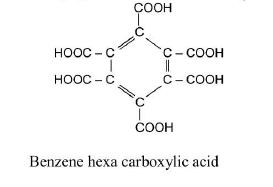
(g) Graphite when heated with oxidising agents like alkaline `KMnO_4` forms metallic acid. See fig.1.
(C) Buck Minster Fullerene :
(a) It has the formula `C_(60)` and is made from interlocking hexagonal and pentagonal rings of carbon atoms.
(b) Such molecules are now thought to exist even in chimney root or candle smoke.
(c) The structure of `C_(60)` is similar to the surface of a football which has also set of interlocking hexagons and pentagons.
(d) Another molecu le `C_(70)` has been recently discovered.
(e) These and similar large carbon molecules are sometimes referred as "bucky balls
(A) Diamond :
(a) Each carbon is linked to another atom and so very closed packing in structure of Diamond.
(b) Density and hardness is very much greater for diamond because of closed packing in diamond due to `sp^3` hybrid and are tetrahedrally arranged around it.
(c) Diamond has sharp cutting edges that's why it is employed in cutting of glass.
(d) Diamond crystals are non conductor of electricity because of not presence of mobile electron.
(e) `1` carat of diamond = `200` mgm.
(f) Diamond powder if consumed is fatal and causes death in minutes.
(B) Graphite :
(a) In graphite carbon are `sp^2` hybridised and due to this carbon exist as hexagonal layer.
(b) Each carbon is lined with `3` carbons and one carbon will be left and form a two dimensional shed Iike structure.
(c) Distance between two layers is very large so no regular bond is formed between two layers. The layers are attached with weak van der Waal force of attraction.
(d) The carbon have unpaired electron so graphite is a good conductor of current.
(e) `C-C` bond length in Graphite is shorter (`1.42` `A^o`) than that of Diamond (`1.54` `A^o`).
(f) Graphite has high melting point so it is employed in manufacture of crucible.
(g) Graphite when heated with oxidising agents like alkaline `KMnO_4` forms metallic acid. See fig.1.
(C) Buck Minster Fullerene :
(a) It has the formula `C_(60)` and is made from interlocking hexagonal and pentagonal rings of carbon atoms.
(b) Such molecules are now thought to exist even in chimney root or candle smoke.
(c) The structure of `C_(60)` is similar to the surface of a football which has also set of interlocking hexagons and pentagons.
(d) Another molecu le `C_(70)` has been recently discovered.
(e) These and similar large carbon molecules are sometimes referred as "bucky balls