Thermodynamic Relationship Between `DeltaG` and Equilibrium Constant :
`Delta G = RT ln (p_2/p_1)`
`G_2 = G_1 + RT ln (p_2/p_1)`
`G_2 = G^o + RT ln (p_2/ (1 b a r))`
Consider a reaction `A+ B -> C`
`G_A = G_A^o + RT ln ((p_A)/(1 b a r))`
`G_B = G_B^o + RT ln ((p_B)/(1 b a r))`
`G_C = G_C^o + RT ln ((p_C)/(1 b a r))`
`Delta G_r = G_r^o + RT ln (p_C)/[((p_A)/(1 b a r)) ((p_b)/(1 b a r))]`
`Delta G_r = G_r^o + RT lnQ [Q = text(Reaction quotient)]`
`Delta G_r = -RT ln K_(eq) ` `(text(At) eq^m, (DeltaG_r)_(T, P) =0)`
`Delta G_r^o = Delta H^o -T Delta S^o`
`ln K_(eq) = (- Delta_r H^o)/(RT) + (Delta_r S^o)/R`
`ln K_1 = (Delta_r S^o)/R - (Delta_r H^o)/(KT_1)`
`ln K_2 = (Delta_r S^o)/R - (Delta_r H^o)/(KT_2)`
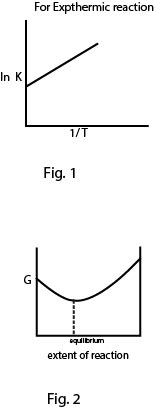
`ln (K_2/K_1) = (Delta H^o)/R(1/T_1 -1/T_2)` (See fig.1)
`ast` `text(For a chemical reaction)` : During a chemical reaction, reaction proceeds in that direction in which `DeltaG < 0` or value of `G` decreases and attains lowest value at equilibrium condition as show in the figure. Moreover as equilibrium condition is changed in either direction, reaction always proceeds towards equilibrium condition. See fig.2.
`text(Clausius Inequality)` : Clausius inequality is another way for measuring the irrversibility and spontaneity of a process. In mathematical as term, it can be expressed as -
`TdS >= q`
`TdS >= dU - w`
at constant temperature & pressure
`d(U - TS + PV) =< 0`
`(H - TS) =< 0`
`dG =< 0`
`G_2 = G_1 + RT ln (p_2/p_1)`
`G_2 = G^o + RT ln (p_2/ (1 b a r))`
Consider a reaction `A+ B -> C`
`G_A = G_A^o + RT ln ((p_A)/(1 b a r))`
`G_B = G_B^o + RT ln ((p_B)/(1 b a r))`
`G_C = G_C^o + RT ln ((p_C)/(1 b a r))`
`Delta G_r = G_r^o + RT ln (p_C)/[((p_A)/(1 b a r)) ((p_b)/(1 b a r))]`
`Delta G_r = G_r^o + RT lnQ [Q = text(Reaction quotient)]`
`Delta G_r = -RT ln K_(eq) ` `(text(At) eq^m, (DeltaG_r)_(T, P) =0)`
`Delta G_r^o = Delta H^o -T Delta S^o`
`ln K_(eq) = (- Delta_r H^o)/(RT) + (Delta_r S^o)/R`
`ln K_1 = (Delta_r S^o)/R - (Delta_r H^o)/(KT_1)`
`ln K_2 = (Delta_r S^o)/R - (Delta_r H^o)/(KT_2)`
`ln (K_2/K_1) = (Delta H^o)/R(1/T_1 -1/T_2)` (See fig.1)
`ast` `text(For a chemical reaction)` : During a chemical reaction, reaction proceeds in that direction in which `DeltaG < 0` or value of `G` decreases and attains lowest value at equilibrium condition as show in the figure. Moreover as equilibrium condition is changed in either direction, reaction always proceeds towards equilibrium condition. See fig.2.
`text(Clausius Inequality)` : Clausius inequality is another way for measuring the irrversibility and spontaneity of a process. In mathematical as term, it can be expressed as -
`TdS >= q`
`TdS >= dU - w`
at constant temperature & pressure
`d(U - TS + PV) =< 0`
`(H - TS) =< 0`
`dG =< 0`