Thermal Equilibrium
Equilibrium in mechanics means that the net external force and torque on a system are zero.
The term 'equilibrium' in thermodynamics appears in a different context : we say the state of a system is an equilibrium state if the macroscopic variables that characterize the system do not change in time.
For example, a gas inside a closed rigid container, completely insulated from its surroundings, with fixed values of pressure, volume, temperature, mass and composition that do not change with time, is in a state of thermodynamic equilibrium.
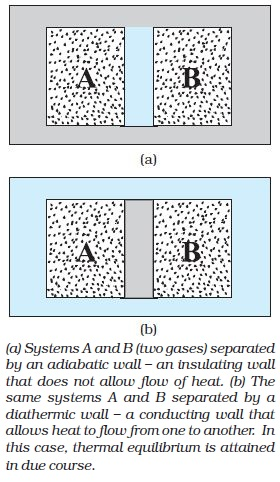
Consider two gases A and B occupying two different containers. Let the pressure and volume of the gases be (`P_A, V_A`) and (`P_B, V_B`) respectively. Suppose first that the two systems are put in proximity but are separated by an adiabatic wall - an insulating wall (can be movable).
The systems are insulated from the rest of the surroundings also by similar adiabatic walls. The situation is shown schematically in Fig-(a).
In this case, it is found that any possible pair of values (`P_A, V_A`) will be in equilibrium with any possible pair of values (`P_B, V_B`).
Next, suppose that the adiabatic wall is replaced by a diathermic wall - a conducting wall that allows energy flow (heat) from one to another. It is then found that the macroscopic variables of the systems A and B change spontaneously until both the systems attain equilibrium states. After that there is no change in their states. [Fig-(b)]
The pressure and volume variables of the two gases change to (`P_B^′ , V_B^′`) and (`P_A^′ , V_A^′` ) such that the new states of A and B are in equilibrium with each other. There is no more energy flow from one to another. We then say that the system A is in thermal equilibrium with the system B.
The term 'equilibrium' in thermodynamics appears in a different context : we say the state of a system is an equilibrium state if the macroscopic variables that characterize the system do not change in time.
For example, a gas inside a closed rigid container, completely insulated from its surroundings, with fixed values of pressure, volume, temperature, mass and composition that do not change with time, is in a state of thermodynamic equilibrium.
Consider two gases A and B occupying two different containers. Let the pressure and volume of the gases be (`P_A, V_A`) and (`P_B, V_B`) respectively. Suppose first that the two systems are put in proximity but are separated by an adiabatic wall - an insulating wall (can be movable).
The systems are insulated from the rest of the surroundings also by similar adiabatic walls. The situation is shown schematically in Fig-(a).
In this case, it is found that any possible pair of values (`P_A, V_A`) will be in equilibrium with any possible pair of values (`P_B, V_B`).
Next, suppose that the adiabatic wall is replaced by a diathermic wall - a conducting wall that allows energy flow (heat) from one to another. It is then found that the macroscopic variables of the systems A and B change spontaneously until both the systems attain equilibrium states. After that there is no change in their states. [Fig-(b)]
The pressure and volume variables of the two gases change to (`P_B^′ , V_B^′`) and (`P_A^′ , V_A^′` ) such that the new states of A and B are in equilibrium with each other. There is no more energy flow from one to another. We then say that the system A is in thermal equilibrium with the system B.