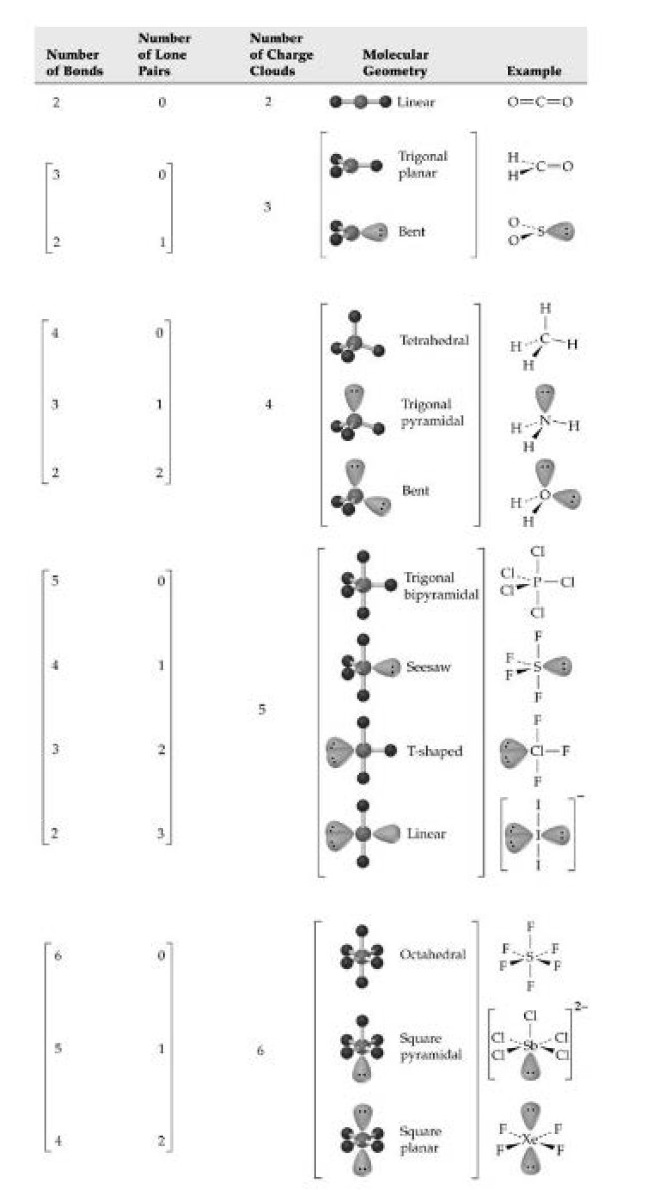
Geometry of Molecules :
For the pred iction of geometrical shapes of molecules with the help of VSEPR theory, it is convenient to divide molecules into two categories as
(i) molecules in which the central atom has no lone pair and
(ii) molecules in which the central atom has one or more lone pairs.
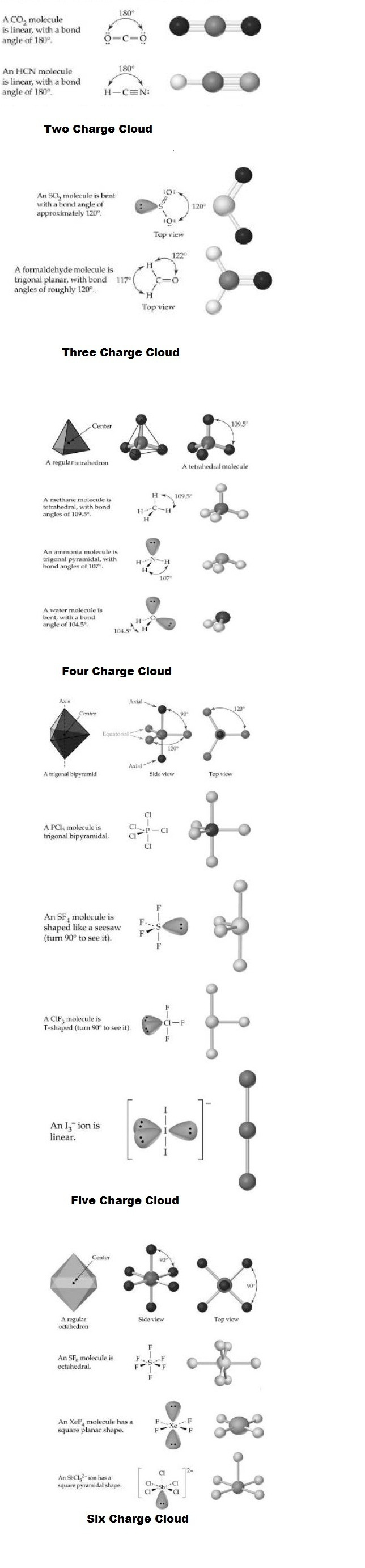
`text(Two Charge Clouds)`: When there are only two charge clouds, as occurs on the carbon atoms of (two double bonds) and `HCN` (one single bond and one triple bond}, the clouds are farthest apart when they point in opposite directions. Thus, `CO_2` and `HCN ` are linear molecules with bond angles of `180^0`.
`text(Three Charge Clouds)` : When there are three charge clouds, as occurs on the carbon atom of formaldehyde (two single bonds and one double bond} and the sulfur atom of `SO_2` (one single bond, one double bond, and one lone pair), the clouds are farthest apart when they lie in the same plane and point to the corners of an equilateral triangle. Thus, a formaldehyde molecule has a trigonal planar shape, with `H- C- H` and `H-C=O` bond angles near `120^o`. Similarly, an `SO_2` molecule has a trigonal planar arrangement of its three charge clouds on sulfur, but one point of thetriangle is occupied by a lone pair and two points by oxygen atoms. The molecule therefore has a bent rather than linear shape, with an `O - S- O` bond angle of approximately `120^o` rather than `180^o`.
`text(Four Charge Clouds)`: When there are four charge clouds, as occurs on the central atoms in `CH_4` (four single bonds), `NH_3` (three single bonds and one lone pair), and `H_2O` (two single bonds and two lone pairs), the clouds are farthest apart if they extend toward the corners of a regular tetrahedron. The central atom lies in the center of the tetrahedron, the charge clouds point toward the four corners, and the angle between two lines drawn from the center to any two corners is `109.5^o`
`text(Five Charge Clouds)`: Five charge clouds, such as are found on the central atoms in `PCI_5, SF_4` and `CIF_3` are oriented toward the corners of a geometric figure called a trigonalbipyramid . Three clouds lie in a plane and point toward the corners of an
equilateral triangle, the fourth cloud points directly up, and the fifth cloud points down:
`text(Six Charge Clouds)`: Six charge clouds around an atom orient toward the six corners of a regular octahedron, a geometric solid whose eight faces are equilateral triangles. All six positions are equivalent, and the angle between any two adjacent positions is `90^0`.
`SF_6` has six bond pairs in the outer shell and is a regular octahedron with bond angles of exactly `90^o`. In `Br F` 5, the `Br` also has six outer pairs of electrons, made up of five bond pairs and one lone pair. The lone pair reduces the bond angles to `84^o30'`.
(i) molecules in which the central atom has no lone pair and
(ii) molecules in which the central atom has one or more lone pairs.
`text(Two Charge Clouds)`: When there are only two charge clouds, as occurs on the carbon atoms of (two double bonds) and `HCN` (one single bond and one triple bond}, the clouds are farthest apart when they point in opposite directions. Thus, `CO_2` and `HCN ` are linear molecules with bond angles of `180^0`.
`text(Three Charge Clouds)` : When there are three charge clouds, as occurs on the carbon atom of formaldehyde (two single bonds and one double bond} and the sulfur atom of `SO_2` (one single bond, one double bond, and one lone pair), the clouds are farthest apart when they lie in the same plane and point to the corners of an equilateral triangle. Thus, a formaldehyde molecule has a trigonal planar shape, with `H- C- H` and `H-C=O` bond angles near `120^o`. Similarly, an `SO_2` molecule has a trigonal planar arrangement of its three charge clouds on sulfur, but one point of thetriangle is occupied by a lone pair and two points by oxygen atoms. The molecule therefore has a bent rather than linear shape, with an `O - S- O` bond angle of approximately `120^o` rather than `180^o`.
`text(Four Charge Clouds)`: When there are four charge clouds, as occurs on the central atoms in `CH_4` (four single bonds), `NH_3` (three single bonds and one lone pair), and `H_2O` (two single bonds and two lone pairs), the clouds are farthest apart if they extend toward the corners of a regular tetrahedron. The central atom lies in the center of the tetrahedron, the charge clouds point toward the four corners, and the angle between two lines drawn from the center to any two corners is `109.5^o`
`text(Five Charge Clouds)`: Five charge clouds, such as are found on the central atoms in `PCI_5, SF_4` and `CIF_3` are oriented toward the corners of a geometric figure called a trigonalbipyramid . Three clouds lie in a plane and point toward the corners of an
equilateral triangle, the fourth cloud points directly up, and the fifth cloud points down:
`text(Six Charge Clouds)`: Six charge clouds around an atom orient toward the six corners of a regular octahedron, a geometric solid whose eight faces are equilateral triangles. All six positions are equivalent, and the angle between any two adjacent positions is `90^0`.
`SF_6` has six bond pairs in the outer shell and is a regular octahedron with bond angles of exactly `90^o`. In `Br F` 5, the `Br` also has six outer pairs of electrons, made up of five bond pairs and one lone pair. The lone pair reduces the bond angles to `84^o30'`.