Work
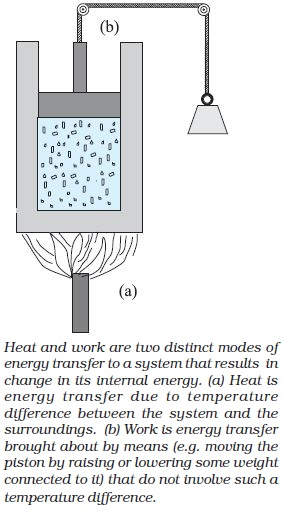
Consider the system to be a certain mass of gas contained in a cylinder with a movable piston as shown in Fig. Experience shows there are two ways of changing the state of the gas (and hence its internal energy).
One way is to put the cylinder in contact with a body at a higher temperature than that of the gas.
The other way is to push the piston down i.e. to do work on the system, which again results in increasing the internal energy of the gas.
In short, heat and work are two different modes of altering the state of a thermodynamic system and changing its internal energy.
Heat and work in thermodynamics are not state variables. They are modes of energy transfer to a system resulting in change in its internal energy.
One way is to put the cylinder in contact with a body at a higher temperature than that of the gas.
The other way is to push the piston down i.e. to do work on the system, which again results in increasing the internal energy of the gas.
In short, heat and work are two different modes of altering the state of a thermodynamic system and changing its internal energy.
Heat and work in thermodynamics are not state variables. They are modes of energy transfer to a system resulting in change in its internal energy.
