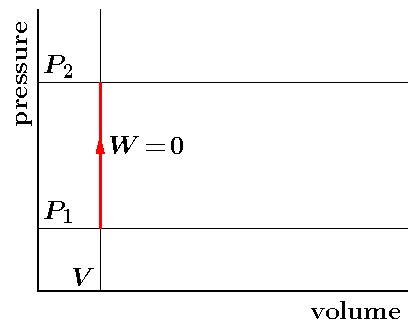
Isobaric Process
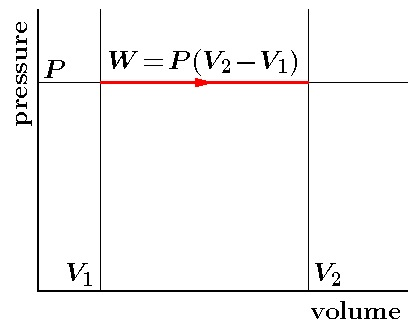
In an isobaric process, P is fixed. Work done by the gas is
`W = P (V_2 - V_1) = mu R (T_2 - T_1)`
Since temperature changes, so does internal energy. The heat absorbed goes partly to increase internal energy and partly to do work.
The change in temperature for a given amount of heat is determined by the specific heat of the gas at constant pressure.
`W = P (V_2 - V_1) = mu R (T_2 - T_1)`
Since temperature changes, so does internal energy. The heat absorbed goes partly to increase internal energy and partly to do work.
The change in temperature for a given amount of heat is determined by the specific heat of the gas at constant pressure.