DIELECTRICS
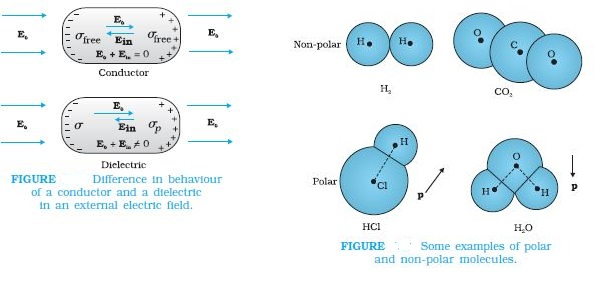
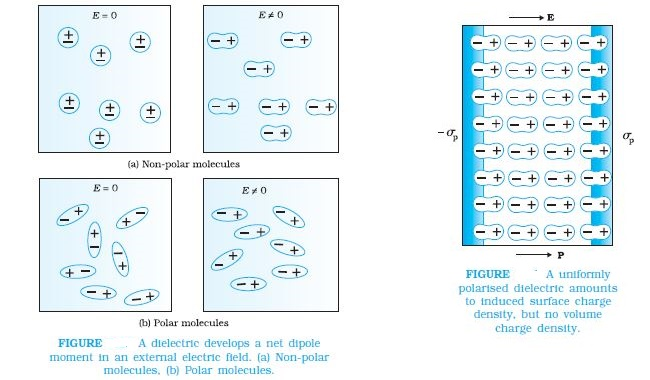
Dielectrics are non-conducting substances. In contrast to conductors, they have no (or negligible number of ) charge carriers. when a conductor is placed in an external electric field. The free charge carriers move and charge distribution in the conductor adjusts itself in such a way that the electric field due to induced charges opposes the external field within the conductor. This happens until, in the static situation, the two fields cancel each other and the net electrostatic field in the conductor is zero. In a dielectric, this free movement of charges is not possible. It turns out that the external field induces dipole moment by stretching or re-orienting
molecules of the dielectric. The collective effect of all the molecular dipole moments is net charges on the surface of the dielectric which produce a field that opposes the external field. Unlike in a conductor, however, the opposing field so induced does not exactly cancel the external field. It only reduces it. The extent of the effect depends on the nature of the dielectric. To understand the
effect, we need to look at the charge distribution of a dielectric at the molecular level.
molecules of the dielectric. The collective effect of all the molecular dipole moments is net charges on the surface of the dielectric which produce a field that opposes the external field. Unlike in a conductor, however, the opposing field so induced does not exactly cancel the external field. It only reduces it. The extent of the effect depends on the nature of the dielectric. To understand the
effect, we need to look at the charge distribution of a dielectric at the molecular level.