Calculation of the Pressure of an Ideal Gas
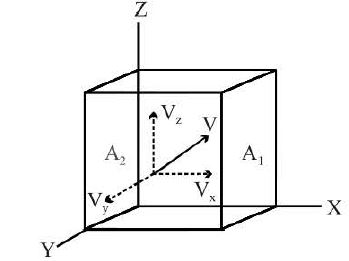
Consider an ideal gas enclosed in a cubical vessel of edge L Take a corner of the vessel as the origin 0 and the X-, Y-, Z- axes along the edges. Let `A_1`, and `A_2`, be the parallel faces perpendicular to the X-ax.is. Consider a molecule moving with velocity `vecv`. The components of the velocity along the axes are `v_x, v_y` and `v_z`. When the molecule collides with the force `A_1`, the x-component of the velocity is reversed whereas the y- and the z-components remain unchanged. This follows from our assumption that the collisions of the molecules with the wall are perfectly elastic. The change in momentum of the molecule is
`Deltap = (- mv_x)- (mv_x)=-2mv_x`
As the momentum remains conserved in a collision, the change in momentum ofthe wall is
`Deltap^'=2mv_x.....(1)`
After rebound, this molecule travels towards `A_2` with the x-component of velocity equal to - `v_x`. Any collision of the molecule with any other face (except for `A_2`) does not change the value of `v_x`. So, it travels between `A_1` and `A_2` with a constant x-component of velocity which is equal to `v_x`. Note that we can neglect any collision with the other molecules.
The distance travelled parallel to the x-direction between `A_1` and `A_2` `= L`. Thus, the time taken by the molecule to go from `A_1` to `A_2` `= L/(v_x)`. The molecule rebounds from `A_2`, travels towards `a_1` and collides with it after another time interval `L//v_x`. Thus, the time between two consecutive collisions of this molecule with `A_1` is `Deltat=2L//v_x` The number of collisions of this molecule with `A_1` in unit time is
`n=1/(Deltat)=(v_x)/(2L)....(2)`
The momentum imparted per unit time to the wall by this molecule is, from (1) and (2),
`DeltaF = nDeltap^'`
`=(v_x)/(2L)xx2mv_x=m/Lv_x^2`
This is also the force exerted on the wall `A_1`, due to this molecule. The total force on the wall `A_1`, due to all the molecules is
`F=Sigmam/Lv_x^2`
`=m/LSigmav_x^2....(3)`
As all directions are equivalent, we have `Sigmav_x^2=Sigmav_y^2=Sigmav_z^2`
`=1/3Sigma(v_x^2+v_y^2+v_z^2)`
`=1/3Sigmav^2`
Thus, from (3), `F=1/3m/LSigmav^2`
If N is the total number of molecules in the sample, we can write
`F=1/3\(mN)/L\(Sigmav^2)/N`
The pressure is force per unit area so that
`p=F/L^2`
`=1/3\(mN)/L^3\(Sigmav^2)/N`
`=1/3\M/L^3\(Sigmav^2)/N=1/3 rho (Sigmav^2)/N`
where M is the total mass of the gas taken and `rho` is its density. Also `Sigmav^2//N` is the average of the speeds squared. It is written as `v^2` and is called mean square speed. Thus, the pressure is
`p=1/3rhov^2`
`pV=1/3Mv^2`
`pV=1/3Nmv^2`
`Deltap = (- mv_x)- (mv_x)=-2mv_x`
As the momentum remains conserved in a collision, the change in momentum ofthe wall is
`Deltap^'=2mv_x.....(1)`
After rebound, this molecule travels towards `A_2` with the x-component of velocity equal to - `v_x`. Any collision of the molecule with any other face (except for `A_2`) does not change the value of `v_x`. So, it travels between `A_1` and `A_2` with a constant x-component of velocity which is equal to `v_x`. Note that we can neglect any collision with the other molecules.
The distance travelled parallel to the x-direction between `A_1` and `A_2` `= L`. Thus, the time taken by the molecule to go from `A_1` to `A_2` `= L/(v_x)`. The molecule rebounds from `A_2`, travels towards `a_1` and collides with it after another time interval `L//v_x`. Thus, the time between two consecutive collisions of this molecule with `A_1` is `Deltat=2L//v_x` The number of collisions of this molecule with `A_1` in unit time is
`n=1/(Deltat)=(v_x)/(2L)....(2)`
The momentum imparted per unit time to the wall by this molecule is, from (1) and (2),
`DeltaF = nDeltap^'`
`=(v_x)/(2L)xx2mv_x=m/Lv_x^2`
This is also the force exerted on the wall `A_1`, due to this molecule. The total force on the wall `A_1`, due to all the molecules is
`F=Sigmam/Lv_x^2`
`=m/LSigmav_x^2....(3)`
As all directions are equivalent, we have `Sigmav_x^2=Sigmav_y^2=Sigmav_z^2`
`=1/3Sigma(v_x^2+v_y^2+v_z^2)`
`=1/3Sigmav^2`
Thus, from (3), `F=1/3m/LSigmav^2`
If N is the total number of molecules in the sample, we can write
`F=1/3\(mN)/L\(Sigmav^2)/N`
The pressure is force per unit area so that
`p=F/L^2`
`=1/3\(mN)/L^3\(Sigmav^2)/N`
`=1/3\M/L^3\(Sigmav^2)/N=1/3 rho (Sigmav^2)/N`
where M is the total mass of the gas taken and `rho` is its density. Also `Sigmav^2//N` is the average of the speeds squared. It is written as `v^2` and is called mean square speed. Thus, the pressure is
`p=1/3rhov^2`
`pV=1/3Mv^2`
`pV=1/3Nmv^2`