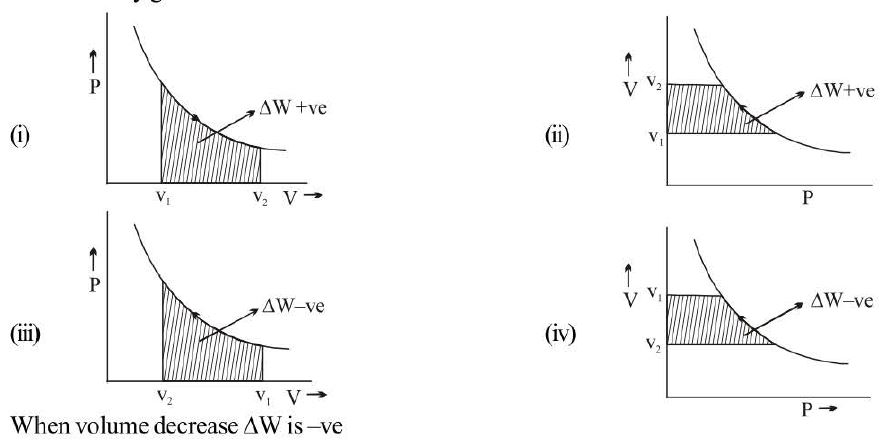
Calculation of Work Done by Gas
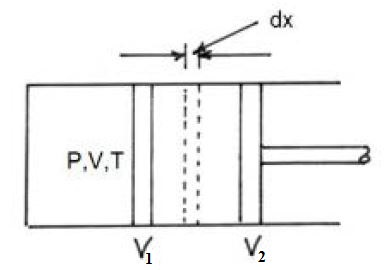
Let P and V be the pressure and volume of a gas. If A be the area of the piston, then force exerted by gas on the piston is,
`F = P xxA`
Let the piston moves 'dx' distance during the expansion of the gas. Work done by gas in the small displacement is
`dW =F dx=P Adx`
Since change in volume is dV = A dx
`dW=PdV`
`W=int_(V_1)^(V_2)PdV`
`F = P xxA`
Let the piston moves 'dx' distance during the expansion of the gas. Work done by gas in the small displacement is
`dW =F dx=P Adx`
Since change in volume is dV = A dx
`dW=PdV`
`W=int_(V_1)^(V_2)PdV`