Carnot Heat Engine
(A) `text(The Engine :)`
As according to the second law of thermodynamics whole of heat can never be converted into work, the question then arises under what conditions the conversion of heat to work is these questions Carnot developed an ideal heat engine which is supposed to consist of the following four components :
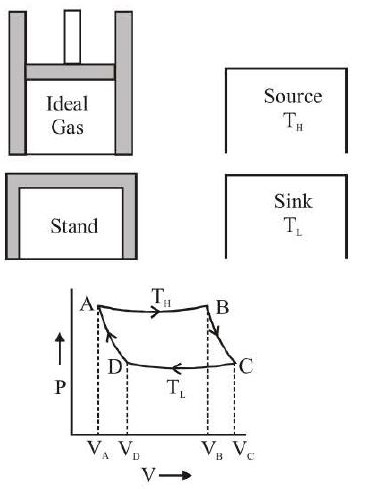
(a) A cylinder with perfectly non-conducting walls and a perfectly conducting base containing a perfect gas as working substance and fitted with a non-conducting frictionless piston.
(b) A source of infinite thermal capacity maintained at constant higher temperature `T_H`.
(c) A sink of infinite thermal capacity maintained at constant lower temperature `T_L`.
(d) A perfectly non-conducting stand for the cylinder.
Here it is worth mentioning that as all the above mentioned components cannot exist in reality, Carnot engine is an ideal (hypothetical) engine which can never be actually constructed.
(B) `text(Carnot Cycle [or Working of the Engine] :)`
The working substance in a Carnot engine is taken through a reversible cycle consisting of the following four steps :
(a) The cylinder containing ideal gas is placed on the source and the gas is allowed to expand slowly at constant temperature `T_H` absorbing heat `Q_H`. This isothermal change is represented by the curve AB in the indicator diagram.
(b) The cylinder is then placed on the non-conducting stand and the gas is allowed to expand adiabatically till the temperature falls from `T_H` to `T_L`. This adiabatic expansion is represented by the curve BC.
(c) The cylinder is next placed on the sink and the gas is compressed at constant temperature `T_L`. This adiabatic expansion is represented by the curve BC.
(iv) Finally the cylinder is again placed on the non-conducting stand and the compression is continued so that the gas returns to its initial stage along DA.
The closed path ABCDA represents the so called Carnot cycle and the four stages taken together represent a cyclic process.
As according to the second law of thermodynamics whole of heat can never be converted into work, the question then arises under what conditions the conversion of heat to work is these questions Carnot developed an ideal heat engine which is supposed to consist of the following four components :
(a) A cylinder with perfectly non-conducting walls and a perfectly conducting base containing a perfect gas as working substance and fitted with a non-conducting frictionless piston.
(b) A source of infinite thermal capacity maintained at constant higher temperature `T_H`.
(c) A sink of infinite thermal capacity maintained at constant lower temperature `T_L`.
(d) A perfectly non-conducting stand for the cylinder.
Here it is worth mentioning that as all the above mentioned components cannot exist in reality, Carnot engine is an ideal (hypothetical) engine which can never be actually constructed.
(B) `text(Carnot Cycle [or Working of the Engine] :)`
The working substance in a Carnot engine is taken through a reversible cycle consisting of the following four steps :
(a) The cylinder containing ideal gas is placed on the source and the gas is allowed to expand slowly at constant temperature `T_H` absorbing heat `Q_H`. This isothermal change is represented by the curve AB in the indicator diagram.
(b) The cylinder is then placed on the non-conducting stand and the gas is allowed to expand adiabatically till the temperature falls from `T_H` to `T_L`. This adiabatic expansion is represented by the curve BC.
(c) The cylinder is next placed on the sink and the gas is compressed at constant temperature `T_L`. This adiabatic expansion is represented by the curve BC.
(iv) Finally the cylinder is again placed on the non-conducting stand and the compression is continued so that the gas returns to its initial stage along DA.
The closed path ABCDA represents the so called Carnot cycle and the four stages taken together represent a cyclic process.