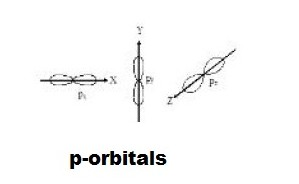
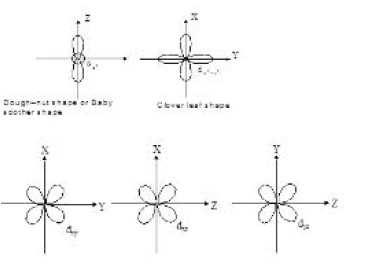
Shapes and size of orbitals

An orbital is the region of space around the nucleus within which the probability of finding an electron of given energy is maximum (B > 90 % ). The shape of this region(electron cloud) gives the shape of the orbital. It is basically determined by the azimuthal quantum number while the orientation of orbital depends on the magnetic quantum number (m). Let us now see the shapes of orbitals in the various subshells.