Determination of Composition in Vapour Phase :
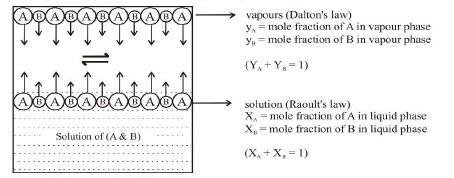
`text(Dalton's Law v/s Raoult's Law)` : The composition of the vapour in equilibrium with the solution can be calculated applying Daltons' law of partial pressures. Let the mole fractions of vapours `A` and `B` be `Y_A` and `Y_B` respectively. Let `P_A` and `P_B` be the partial pressure of vapours `A` and `B` respectively and total pressure `P`.
From Raoult's law, `P = P_A^oX_A + P_B^oX_B`
`P_A = P_A^oX_A` and `P_B = P_B^oX_B`
From Dalton's law,
Partial pressure = Mole fraction `xx` Total pressure
For `A`, `P_A = y_A xx P = - P_A^oX_A`
`=> y_A = (P_A^oX_A)/P......................(1)`
Similarly
`=> y_B = (P_B^oX_B)/P.............................(2)`
Above formula is used for calculation of mole fraction of `B` in vapour phase
On adding `X_A + X_B = (P xx y_A)/(P_A^o) + (P xx y_B)/(P_B^o) =1`
`=> 1/P = y_A/P_B^o + y_B/P_B^o....................................(3)`
Above formula is used to calculate total vapour pressure when mole fractions are given in vapoure phase.
`P = P_A^oX_A + P_B^oX_B =>` This formula is used to calculate total pressure when mole fraction are given in liquid phase
`1/P = y_A/(P_A^o) + y_B/(P_B^o) => ` This formula is used to calculate total pressure when mole fraction are given in vapour phase.
Thus, in case of ideal solution the vapour phase is richer with more volatile component i.e., the one having relatively greater vapour pressure.
From Raoult's law, `P = P_A^oX_A + P_B^oX_B`
`P_A = P_A^oX_A` and `P_B = P_B^oX_B`
From Dalton's law,
Partial pressure = Mole fraction `xx` Total pressure
For `A`, `P_A = y_A xx P = - P_A^oX_A`
`=> y_A = (P_A^oX_A)/P......................(1)`
Similarly
`=> y_B = (P_B^oX_B)/P.............................(2)`
Above formula is used for calculation of mole fraction of `B` in vapour phase
On adding `X_A + X_B = (P xx y_A)/(P_A^o) + (P xx y_B)/(P_B^o) =1`
`=> 1/P = y_A/P_B^o + y_B/P_B^o....................................(3)`
Above formula is used to calculate total vapour pressure when mole fractions are given in vapoure phase.
`P = P_A^oX_A + P_B^oX_B =>` This formula is used to calculate total pressure when mole fraction are given in liquid phase
`1/P = y_A/(P_A^o) + y_B/(P_B^o) => ` This formula is used to calculate total pressure when mole fraction are given in vapour phase.
Thus, in case of ideal solution the vapour phase is richer with more volatile component i.e., the one having relatively greater vapour pressure.