Elevation in Boiling Point :
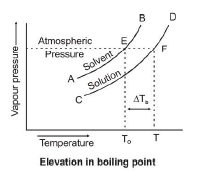
The boiling points elevates when a non-volatile solute is added to a volatile solvent which occurs due to lowering of vapour pressure. The boiling point of a liquid may be defined as the temperature at which its vapour pressure becomes equal to atmospheric pressure.
So, when a non-volatile solute is added to a volatile solvent results lowering of vapour pressure and consequent elevation of boiling point.
where
`DeltaT_b = T_b - T_b^o`
`DeltaT_b =` elevation in B.P.
`Delta P =` lowering of V.P.
`X_B =` mole fraction of solute
`K =` elevation constant
`T_b^o =` boiling point of solvent
`T_b =` boiling point of solution
It is found that elevation of boiling point is directly proportional to the number of moles of the solute in given amount of the solvent(`m`).
`Delta T_b prop m`
`DeltaT_b prop K_b m`
Where 'm' is the molality of solution.
Where `K_b` is ebullioscopic or boiling point elevation constant. When molality of the solution is equal to one, then
`DeltaT_b = K_b`
Hence molal elevation constant of the solvent may be defined as the elevation in its boiling point when one mole of non-volatile solute is dissolved per kg (`1000` gm) of solvent. The unit of `K_b` are `K` `kg` `mol^(-1)`.
Because molality of solutron `m = W_B/M_B * 1000/W_A`
So `Delta T_b =K_b W_B/M_B * 1000/W_A` or `M_B = (1000 xx K_b xx W_B)/(DeltaT_b xx W_A)`
Where `W_A=` mass of solvent, `W_B =` mass of solute,
`M_A =` Molar mass of solvent, `M_B =` Molar mass of solute.
So, when a non-volatile solute is added to a volatile solvent results lowering of vapour pressure and consequent elevation of boiling point.
where
`DeltaT_b = T_b - T_b^o`
`DeltaT_b =` elevation in B.P.
`Delta P =` lowering of V.P.
`X_B =` mole fraction of solute
`K =` elevation constant
`T_b^o =` boiling point of solvent
`T_b =` boiling point of solution
It is found that elevation of boiling point is directly proportional to the number of moles of the solute in given amount of the solvent(`m`).
`Delta T_b prop m`
`DeltaT_b prop K_b m`
Where 'm' is the molality of solution.
Where `K_b` is ebullioscopic or boiling point elevation constant. When molality of the solution is equal to one, then
`DeltaT_b = K_b`
Hence molal elevation constant of the solvent may be defined as the elevation in its boiling point when one mole of non-volatile solute is dissolved per kg (`1000` gm) of solvent. The unit of `K_b` are `K` `kg` `mol^(-1)`.
Because molality of solutron `m = W_B/M_B * 1000/W_A`
So `Delta T_b =K_b W_B/M_B * 1000/W_A` or `M_B = (1000 xx K_b xx W_B)/(DeltaT_b xx W_A)`
Where `W_A=` mass of solvent, `W_B =` mass of solute,
`M_A =` Molar mass of solvent, `M_B =` Molar mass of solute.