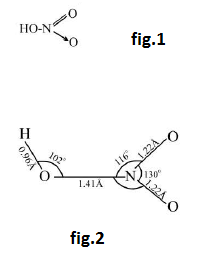
Chemical Properties :
(a) It is very strong acid. It exhibits usual properties of acids. It reacts with basic oxides, carbonates, bicarbonates and hydroxides forming corresponding salts.
`CaO + 2HNO_3 -> Ca(NO_3)_2 + H_2O`
`Na_2CO_3 +2HNO_3 -> 2NaNO_3 +H_2O +CO_2`
`NaOH +HNO_3 -> NaNO_3 +H_2O`
`(b)` `text(Oxidising nature :)` Nitric acid acts as a strong oxidising agent as it decomposes to give nascent oxygen easily.
`2HNO_3 -> H_2O + 2NO_2 + O`
or `2HNO_3 -> H_2O + 2NO + 3O`
(i) `text(Oxidation of non-metals :)` The nascent oxygen oxidises various non-metals to their corresponding highest oxyacids.
`ast` Sulphur is oxidises to sulphuric acid
`S + undersettext(conc. & hot)(6HNO_3) -> H_2SO_4 + 6NO_2 + 2H_2O`
`ast` Carbon is oxidised to carbonic acid
`C + 4HNO_3 -> H_2CO_3 + 4NO_2 + 2H_2O`
`ast` Phosphorus is oxidised to orthophosphoric acid.
`2P + undersettext(conc. and hot)(10 HNO_3) -> 2H_3PO_4 + 10NO_2 + 2H_2O`
`ast` Iodine is oxidised to iodic acid
`I_2 + undersettext(conc. and hot)(10HNO_3) -> 2HIO_3 + 10NO_2 + 4H_2O`
(ii) `text(Oxidation of metalloids :)` Metalloids like non-metals also form highest oxyacids
`ast` Arsenic is oxidised to arsenic acid
`2As + 10HNO_3 -> 2H_3AsO_4 + 10NO_2 +2H_2O`
or `As + undersettext(conc. and hot)(5HNO_3) -> H_3AsO_4 + 5NO_2 + H_2O`
`ast` Antimony is oxidised to antimonic acid
`Sb + undersettext(conc. and hot)(5HNO_3) -> H_3SbO_4 + 5NO_2 + H_2O`
`ast` Tin is oxidised to meta-stannic acid.
`Sn + 2HNO_3 -> H_2SnO_3 + 4NO_2 + H_2O`
(iii) `text(Oxidation of Compounds :)`
`ast` Sulphur dioxide is oxidised to sulphuric acid
`SO_2 + 2HNO_3 -> H_2SO_4 + 2NO_2`
`ast` Hydrogen sulphiode is oxidised to sulphur
`H_2S + 2HNO_3 -> 2NO_2 + 2H_2O + S`
`ast` Ferrous sulphate is oxidised to ferric sulphate in presence of `H_2SO_4`
`6FeSO_4 + 3H_2SO_4 + 2HNO_3 -> 3Fe_2(SO_4)_3 + 2NO + 4H_2O`
`ast` Iodine is liberated from `KI`.
`6KI + 8HNO_3 -> 6KNO_3 + 2NO + 3I_2 + 4H_2O`
`ast` `HBr`, `HI` are oxidised to `Br_2` and `I_2,` respectively.
`2HBr + 2HNOI_3 -> Br_2 + 2NO_2 + 2H_2O`
Similarly, `2HI + 2HNO_3 -> I_2 + 2NO_2 + 2H_2O`
`ast` Ferrous sulphide is oxidised to ferrous sulphate
`FeS + HNO_3 -> Fe_2(SO_4)_3 + 8NO_2 + 4H_2O`
`ast` Stannous chloride is oxidised to stannic chloride is presence of `HCl`.
`2HNO_3 + 14H -> undersettext(Hydroxylamine)(NH_2OH) + NH_3 + 5H_2O`
`ul(NH_3 + HNO_3 -> NH_4NO_3)`
`7SnCl_2 + 14HCl + 3HNO_3 -> 7SnCl_4 + NH_2OH + NH_4NO_3 + 5H_2O`
`ast` Cane sugar is oxidised to oxalic acid.
`C_(12)H_(22)O_(11) + 36HNO_3 -> 6(COOH)_2 + 36NO_2 + 23H_2O`
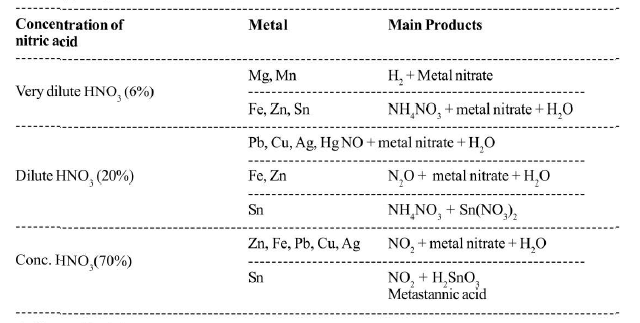
`(c)` `text(Action on Metals :)` Most of the metals will the exception of noble metals like gold and platinum are attacked by Nitric acid plays a double role in the action of metals, i,e, it acts as an acid as well as an oxidising agent. Armstrong postulated that primary action of nitric acid is to produce hydrogen in the nascent form. Before this hydrogen is allowed to escape, it reduces the nitric acid into number of products like `NO_2, NO, N_2O, N_2` or `NH_3` according to the following reactions:
Metal `+ HNO_3 ->` Nitrate + H
`2HNO_3 + 2H -> 2NO + 2H_2O`
`2HNO_3 + 6H -> 2NO + 4H_2O`
`2HNO_3 + 10H -> N_2 + 6H_2O`
`2HNO_3 + 16 H -> 2NH_3 + 6H_2O`
The progress of the reaction is controlled by a number of factors:
(a) the nature of the metal,
(b) the concentration of the acid,
(c) the temperature of the reaction,
(d) the presence of other impurities.
See Table.
`text(Action on Proteins)`
(i) Nitric acid attacks proteins forming a yellow nitro compound called xanthoprotein. It, therefore, stains skin and renders wool yellow. This property is utilized for the test of proteins.
(ii) `text(Oxidation)` A number of organic compounds are oxidised. Sawdust catches fire when nitric acid is poured on it. Turpentine oil bursts into flames when treated with fuming nitric acid. Cane sugar is oxidised to oxalic acid. Toluene is oxidised to benzoic acid with dil. `HNO_3`
`CaO + 2HNO_3 -> Ca(NO_3)_2 + H_2O`
`Na_2CO_3 +2HNO_3 -> 2NaNO_3 +H_2O +CO_2`
`NaOH +HNO_3 -> NaNO_3 +H_2O`
`(b)` `text(Oxidising nature :)` Nitric acid acts as a strong oxidising agent as it decomposes to give nascent oxygen easily.
`2HNO_3 -> H_2O + 2NO_2 + O`
or `2HNO_3 -> H_2O + 2NO + 3O`
(i) `text(Oxidation of non-metals :)` The nascent oxygen oxidises various non-metals to their corresponding highest oxyacids.
`ast` Sulphur is oxidises to sulphuric acid
`S + undersettext(conc. & hot)(6HNO_3) -> H_2SO_4 + 6NO_2 + 2H_2O`
`ast` Carbon is oxidised to carbonic acid
`C + 4HNO_3 -> H_2CO_3 + 4NO_2 + 2H_2O`
`ast` Phosphorus is oxidised to orthophosphoric acid.
`2P + undersettext(conc. and hot)(10 HNO_3) -> 2H_3PO_4 + 10NO_2 + 2H_2O`
`ast` Iodine is oxidised to iodic acid
`I_2 + undersettext(conc. and hot)(10HNO_3) -> 2HIO_3 + 10NO_2 + 4H_2O`
(ii) `text(Oxidation of metalloids :)` Metalloids like non-metals also form highest oxyacids
`ast` Arsenic is oxidised to arsenic acid
`2As + 10HNO_3 -> 2H_3AsO_4 + 10NO_2 +2H_2O`
or `As + undersettext(conc. and hot)(5HNO_3) -> H_3AsO_4 + 5NO_2 + H_2O`
`ast` Antimony is oxidised to antimonic acid
`Sb + undersettext(conc. and hot)(5HNO_3) -> H_3SbO_4 + 5NO_2 + H_2O`
`ast` Tin is oxidised to meta-stannic acid.
`Sn + 2HNO_3 -> H_2SnO_3 + 4NO_2 + H_2O`
(iii) `text(Oxidation of Compounds :)`
`ast` Sulphur dioxide is oxidised to sulphuric acid
`SO_2 + 2HNO_3 -> H_2SO_4 + 2NO_2`
`ast` Hydrogen sulphiode is oxidised to sulphur
`H_2S + 2HNO_3 -> 2NO_2 + 2H_2O + S`
`ast` Ferrous sulphate is oxidised to ferric sulphate in presence of `H_2SO_4`
`6FeSO_4 + 3H_2SO_4 + 2HNO_3 -> 3Fe_2(SO_4)_3 + 2NO + 4H_2O`
`ast` Iodine is liberated from `KI`.
`6KI + 8HNO_3 -> 6KNO_3 + 2NO + 3I_2 + 4H_2O`
`ast` `HBr`, `HI` are oxidised to `Br_2` and `I_2,` respectively.
`2HBr + 2HNOI_3 -> Br_2 + 2NO_2 + 2H_2O`
Similarly, `2HI + 2HNO_3 -> I_2 + 2NO_2 + 2H_2O`
`ast` Ferrous sulphide is oxidised to ferrous sulphate
`FeS + HNO_3 -> Fe_2(SO_4)_3 + 8NO_2 + 4H_2O`
`ast` Stannous chloride is oxidised to stannic chloride is presence of `HCl`.
`2HNO_3 + 14H -> undersettext(Hydroxylamine)(NH_2OH) + NH_3 + 5H_2O`
`ul(NH_3 + HNO_3 -> NH_4NO_3)`
`7SnCl_2 + 14HCl + 3HNO_3 -> 7SnCl_4 + NH_2OH + NH_4NO_3 + 5H_2O`
`ast` Cane sugar is oxidised to oxalic acid.
`C_(12)H_(22)O_(11) + 36HNO_3 -> 6(COOH)_2 + 36NO_2 + 23H_2O`
`(c)` `text(Action on Metals :)` Most of the metals will the exception of noble metals like gold and platinum are attacked by Nitric acid plays a double role in the action of metals, i,e, it acts as an acid as well as an oxidising agent. Armstrong postulated that primary action of nitric acid is to produce hydrogen in the nascent form. Before this hydrogen is allowed to escape, it reduces the nitric acid into number of products like `NO_2, NO, N_2O, N_2` or `NH_3` according to the following reactions:
Metal `+ HNO_3 ->` Nitrate + H
`2HNO_3 + 2H -> 2NO + 2H_2O`
`2HNO_3 + 6H -> 2NO + 4H_2O`
`2HNO_3 + 10H -> N_2 + 6H_2O`
`2HNO_3 + 16 H -> 2NH_3 + 6H_2O`
The progress of the reaction is controlled by a number of factors:
(a) the nature of the metal,
(b) the concentration of the acid,
(c) the temperature of the reaction,
(d) the presence of other impurities.
See Table.
`text(Action on Proteins)`
(i) Nitric acid attacks proteins forming a yellow nitro compound called xanthoprotein. It, therefore, stains skin and renders wool yellow. This property is utilized for the test of proteins.
(ii) `text(Oxidation)` A number of organic compounds are oxidised. Sawdust catches fire when nitric acid is poured on it. Turpentine oil bursts into flames when treated with fuming nitric acid. Cane sugar is oxidised to oxalic acid. Toluene is oxidised to benzoic acid with dil. `HNO_3`