Classification of Polymers :
Polymers are classified in following ways :
(A) `text(CLASSIFICATION BASED UPON SOURCE :)`
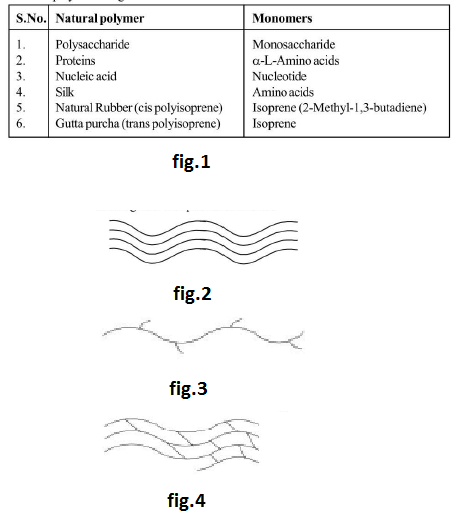
(i) `text(Natural polymers :)` Polymers which are obtained from animals and plants are known as natural polymers. Examples of natural polymers are given below : See fig.1.
Natural polymers which take part in metabolic processes are known as biopolymers. Examples are polysaccharides, proteins, RNA and DNA.
(ii) `text(Semisynthetic polymers :)` Polymers which are prepared from natural polymers are known as semisynthetic polymers. Most of the semisynthetic polymers are prepared from cellulose. Examples are : cellulose acetate, cellulose nitrate, cellulose xanthate and Rayon.
(iii) `text(Synthetic polymers :)` Man-made polymers, i.e. polymers prepared in laboratory are known as synthetic polymers. Example are : PVC, polyethylene, polystyrene, nylon-6, nylon-6, 6, nylon-610, terylene, synthetic rubbers etc.
(B) `text(CLASSIFICATION BASED UPON SHAPE :)`
(i) `text(Linear polymers :)` Polymer whose structure is linear is known as linear polymer. The various linear polymeric chains are stacked over one another to give a well packed structure. See fig.2.
The chains are highly ordered with respect to one another. The structure is close packed in nature, due to which they have high densities, high melting point and high tensile (pulling) strength. Linear polymers can be converted into fibres.
`text(Notes :)`
(a) All fibers are linear polymers. Examples are cellulose, silk, nylon, terylene etc.
(b) Linear polymers may be condensation as well as addition polymers. Examples are cellulose, polypeptide, nucleic acid, nylon, terylene etc.
(ii) `text(Branched chain polymers :)` Branched chain polymers are those in which the monomeric units constitute a branched chain. Due to the presence of branches, these polymers do not pack well. As a result branched chain polymers have lower melting points, low densities and tensile strength as compared to linear polymers.
Branched chain polymers may be formed due to addition as well as condensation polymerisation. Examples are amylopectin, glycogen, low density polyethylene and all vulcanised rubbers. See fig.3.
(iii) `text(Cross-linked or Three Dimensional network polymers :)` In these polymers the initially formed linear polymeric chains are joined together to form a three dimensional network structure. These polymers are hard, rigid and brittle. Cross-linked polymers are always condensation polymers. Resins are cross linked polymers. See fig.4.
(A) `text(CLASSIFICATION BASED UPON SOURCE :)`
(i) `text(Natural polymers :)` Polymers which are obtained from animals and plants are known as natural polymers. Examples of natural polymers are given below : See fig.1.
Natural polymers which take part in metabolic processes are known as biopolymers. Examples are polysaccharides, proteins, RNA and DNA.
(ii) `text(Semisynthetic polymers :)` Polymers which are prepared from natural polymers are known as semisynthetic polymers. Most of the semisynthetic polymers are prepared from cellulose. Examples are : cellulose acetate, cellulose nitrate, cellulose xanthate and Rayon.
(iii) `text(Synthetic polymers :)` Man-made polymers, i.e. polymers prepared in laboratory are known as synthetic polymers. Example are : PVC, polyethylene, polystyrene, nylon-6, nylon-6, 6, nylon-610, terylene, synthetic rubbers etc.
(B) `text(CLASSIFICATION BASED UPON SHAPE :)`
(i) `text(Linear polymers :)` Polymer whose structure is linear is known as linear polymer. The various linear polymeric chains are stacked over one another to give a well packed structure. See fig.2.
The chains are highly ordered with respect to one another. The structure is close packed in nature, due to which they have high densities, high melting point and high tensile (pulling) strength. Linear polymers can be converted into fibres.
`text(Notes :)`
(a) All fibers are linear polymers. Examples are cellulose, silk, nylon, terylene etc.
(b) Linear polymers may be condensation as well as addition polymers. Examples are cellulose, polypeptide, nucleic acid, nylon, terylene etc.
(ii) `text(Branched chain polymers :)` Branched chain polymers are those in which the monomeric units constitute a branched chain. Due to the presence of branches, these polymers do not pack well. As a result branched chain polymers have lower melting points, low densities and tensile strength as compared to linear polymers.
Branched chain polymers may be formed due to addition as well as condensation polymerisation. Examples are amylopectin, glycogen, low density polyethylene and all vulcanised rubbers. See fig.3.
(iii) `text(Cross-linked or Three Dimensional network polymers :)` In these polymers the initially formed linear polymeric chains are joined together to form a three dimensional network structure. These polymers are hard, rigid and brittle. Cross-linked polymers are always condensation polymers. Resins are cross linked polymers. See fig.4.