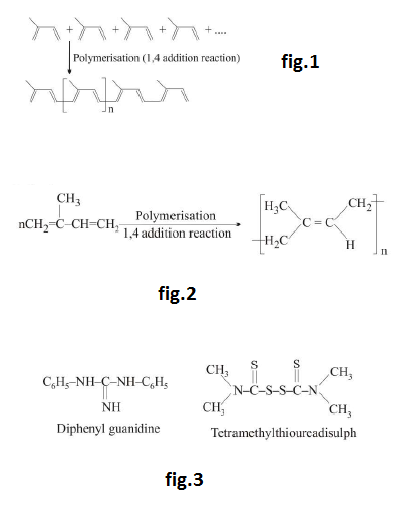
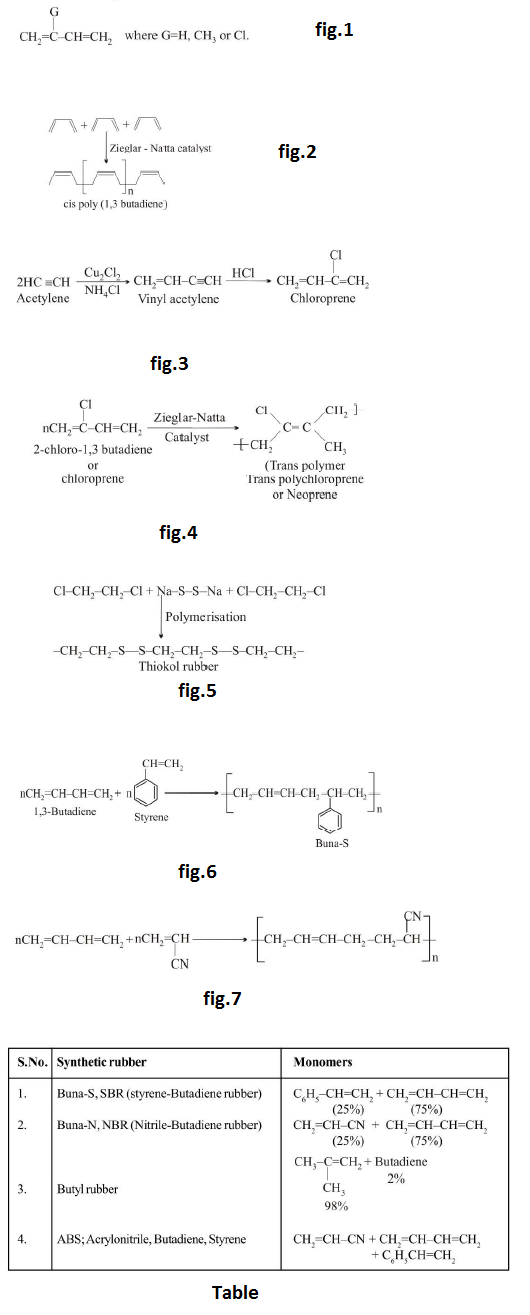
Classification Based on Intermolecular Forces (Secondary Forces) :
(i) Intermolecular forces present between polymeric chains are
(a) Van der Waals forces
(b) Hydrogen bonds and
(c) Dipole- dipole attractions.
(ii) Mechanical properties such as tensile strength, elasticity, toughness etc. depend upon the secondary forces present between the polymeric chains.
(iii) Magnitude of secondary forces depends upon the size of the molecule and the number of functional groups along the polymeric chains.
Magnitude of secondary forces is directly proportional to the length of the polymeric chain. On the basis of magnitude of secondary forces, polymers can be divided into the following five categories :
(a) `text(Elastomers :)` An elastomer is a plastic that stretches and then reverts back to its original shape. It is randomly oriented amorphous polymer. It must have some cross-links so that the chains do not slip over one another. Very weak Van der Waal forces are present in between polymeric chains.
When elastomers are stretched, the random chains stretch out, but there are insufficient Van der Waal forces to maintain them in that configuration and position. When the stretching force is removed, they go back to their random shape. Elastomers have the ability to stretch out over ten times their normal length. Important examples are vulcanized rubbers.
`text(Note :)` Addition polymers obtained from butadiene and its derivatives are elastomers.
(b) `text(Fibres :)` Fibres are linear polymers in which the individual chains of a polymer are held together by hydrogen bonds and/or dipole-dipole attraction. In the fibres, the polymeric chains are highly ordered with respect to one another.
Due to strong intermolecular forces of attraction and highly ordered geometry, fibres have high tensile strength and least elasticity. They have crystalline character and have high melting points and low solubility. Examples are cellulose, nylon, terylene, wool, silk etc.
`text(Note :)`
(i) Condensation polymers formed from bifunctional monomers are fibres in character.
(ii) Addition polymers of alkene derivatives having strong-I group are fibres in character.
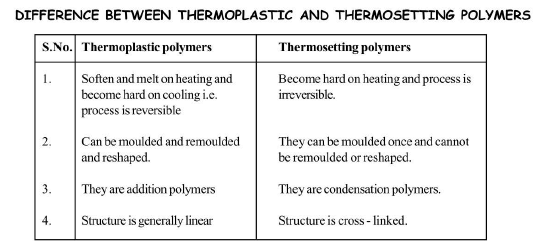
(c) `text(Thermoplastic Polymers :)` Thermoplastic polymers are polymers that have both ordered crystalline regions (the regions of the polymer in which the chains are highly ordered with respect to one another) and amorphous, non-crystalline regions (the regions of the polymer in which the chains are randomly oriented).
The intermolecular forces of attraction are in between elastomers and fibres. There are no cross-links between the polymeric chains. Thermoplastic polymers are hard at room temperature, but when they are heated, the individual chains can slip past one another and the polymer become soft and viscus. This soft and viscous material become rigid on cooling. The process of heating, softening and cooling can be repeated as many times as desired without any change in chemical composition and mechanical properties of the plastic. As a result, these plastics can be moulded into toys, buckets, telephone and television cases. Some common examples are : polyethene, polypropylene, polystyrene, polyvinyl chloride, teflon etc.
`text(Note :)` Addition polymers obtained from ethylene and ethylene derivatives are thermoplastic polymers.
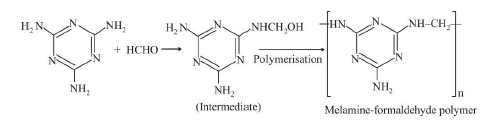
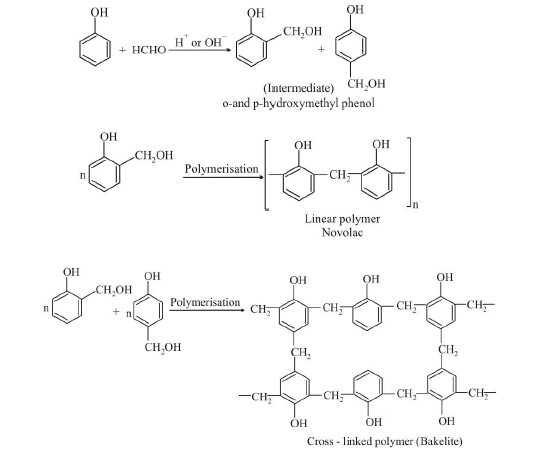
(d) `text(Thermosetting Polymers :)` Polymers which become hard on heating are called thermosetting polymers. Thermosetting polymers can be heated only once when it permanently sets into a solid, which cannot be remelted by heating. Thermosetting polymers are cross-linked polymers. Greater the degree of cross-linking that exist, the more rigid is the polymer. Cross-linking reduces the mobility of the polymer chains, causing them to be relatively brittle materials. The hardening on heating is due to the extensive cross-linking between different polymer chains to give a three dimensional network solid. Examples are : phenol formaldehyde resin, urea-formaldehyde resin, melamine-formaldehyde resin.
See Table.
(a) Van der Waals forces
(b) Hydrogen bonds and
(c) Dipole- dipole attractions.
(ii) Mechanical properties such as tensile strength, elasticity, toughness etc. depend upon the secondary forces present between the polymeric chains.
(iii) Magnitude of secondary forces depends upon the size of the molecule and the number of functional groups along the polymeric chains.
Magnitude of secondary forces is directly proportional to the length of the polymeric chain. On the basis of magnitude of secondary forces, polymers can be divided into the following five categories :
(a) `text(Elastomers :)` An elastomer is a plastic that stretches and then reverts back to its original shape. It is randomly oriented amorphous polymer. It must have some cross-links so that the chains do not slip over one another. Very weak Van der Waal forces are present in between polymeric chains.
When elastomers are stretched, the random chains stretch out, but there are insufficient Van der Waal forces to maintain them in that configuration and position. When the stretching force is removed, they go back to their random shape. Elastomers have the ability to stretch out over ten times their normal length. Important examples are vulcanized rubbers.
`text(Note :)` Addition polymers obtained from butadiene and its derivatives are elastomers.
(b) `text(Fibres :)` Fibres are linear polymers in which the individual chains of a polymer are held together by hydrogen bonds and/or dipole-dipole attraction. In the fibres, the polymeric chains are highly ordered with respect to one another.
Due to strong intermolecular forces of attraction and highly ordered geometry, fibres have high tensile strength and least elasticity. They have crystalline character and have high melting points and low solubility. Examples are cellulose, nylon, terylene, wool, silk etc.
`text(Note :)`
(i) Condensation polymers formed from bifunctional monomers are fibres in character.
(ii) Addition polymers of alkene derivatives having strong-I group are fibres in character.
(c) `text(Thermoplastic Polymers :)` Thermoplastic polymers are polymers that have both ordered crystalline regions (the regions of the polymer in which the chains are highly ordered with respect to one another) and amorphous, non-crystalline regions (the regions of the polymer in which the chains are randomly oriented).
The intermolecular forces of attraction are in between elastomers and fibres. There are no cross-links between the polymeric chains. Thermoplastic polymers are hard at room temperature, but when they are heated, the individual chains can slip past one another and the polymer become soft and viscus. This soft and viscous material become rigid on cooling. The process of heating, softening and cooling can be repeated as many times as desired without any change in chemical composition and mechanical properties of the plastic. As a result, these plastics can be moulded into toys, buckets, telephone and television cases. Some common examples are : polyethene, polypropylene, polystyrene, polyvinyl chloride, teflon etc.
`text(Note :)` Addition polymers obtained from ethylene and ethylene derivatives are thermoplastic polymers.
(d) `text(Thermosetting Polymers :)` Polymers which become hard on heating are called thermosetting polymers. Thermosetting polymers can be heated only once when it permanently sets into a solid, which cannot be remelted by heating. Thermosetting polymers are cross-linked polymers. Greater the degree of cross-linking that exist, the more rigid is the polymer. Cross-linking reduces the mobility of the polymer chains, causing them to be relatively brittle materials. The hardening on heating is due to the extensive cross-linking between different polymer chains to give a three dimensional network solid. Examples are : phenol formaldehyde resin, urea-formaldehyde resin, melamine-formaldehyde resin.
See Table.