Properties of Rays
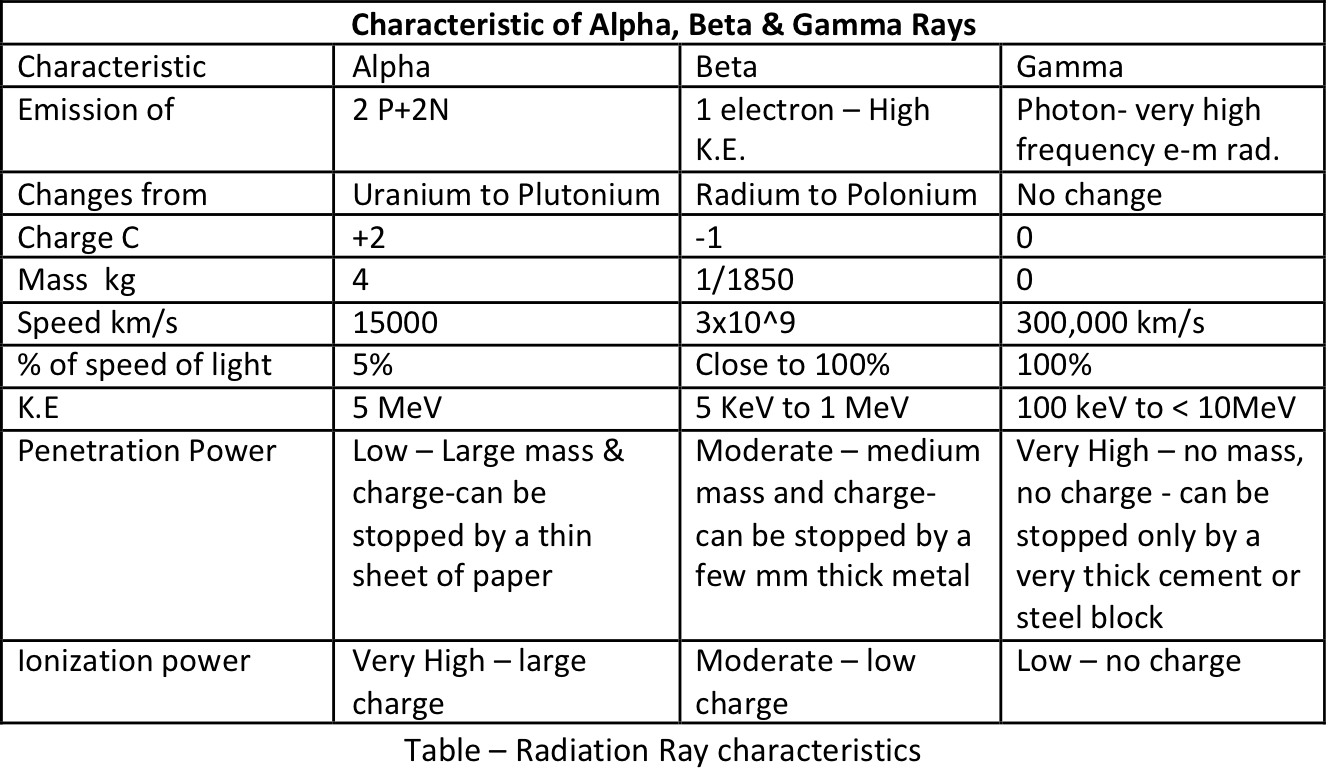
=> Alpha particles have a velocity of `~(1/10)^(th) - (1/20)^(th)` of the speed of light (a very high velocity helium nucleus).
=> Alpha particles have the greatest kinetic energy, because, although they have the slowest speed of the three radiations, they have by far the greatest mass, and this makes all the difference.
=> Compared to beta particles, alpha particles have a speed of, at the least, 18 times less than beta radiation, the mass of an alpha particle is 7400 times greater ` (4 - 1/1850)` than that of a beta particle.
=> Although alpha particles have the largest kinetic energy, they have the least penetrating power because of the larger mass, and, especially, the double positive charge (+2). These fast moving positive electric fields will strongly interact with the negative electrons of the atoms the alpha radiation is passing through so it gets slowed down as it loses its kinetic energy.
=> As argued above, although the alpha particles have the slowest velocity, their greater mass and higher charge enable an alpha particle to 'knock off' electrons from atoms the most easily, i.e. the greatest ionising power.
The doubly positively charged alpha particle will attract and abstract two electrons from atoms/molecules hit forming new ions.
=> Alpha particles have the greatest kinetic energy, because, although they have the slowest speed of the three radiations, they have by far the greatest mass, and this makes all the difference.
=> Compared to beta particles, alpha particles have a speed of, at the least, 18 times less than beta radiation, the mass of an alpha particle is 7400 times greater ` (4 - 1/1850)` than that of a beta particle.
=> Although alpha particles have the largest kinetic energy, they have the least penetrating power because of the larger mass, and, especially, the double positive charge (+2). These fast moving positive electric fields will strongly interact with the negative electrons of the atoms the alpha radiation is passing through so it gets slowed down as it loses its kinetic energy.
=> As argued above, although the alpha particles have the slowest velocity, their greater mass and higher charge enable an alpha particle to 'knock off' electrons from atoms the most easily, i.e. the greatest ionising power.
The doubly positively charged alpha particle will attract and abstract two electrons from atoms/molecules hit forming new ions.