Photoelectric Effect
The photoelectric effect is process where electrons are ejected from a surface by the action oflight (electromagnetic radiation). The process was discovered by Heinrich Hertz in 1887. Attempts to explain the effect by classical electromagnetic failed. In 1905, Albert Einstein presented an explanation based on the quantum concept of Max Planck.
Observation of the experiments on Photo-Electric Effect:
(i) The emission of photoelectrons is instantaneous.
(ii) the number of photoelectrons emitted per second is proportional to the intensity of the incident light.
(iii) The maximum velocity with which electrons emerge is dependent only on the frequency and not on the intensity of the incident light.
(iv) There is always a lower limit of frequency called threshold frequency below which no emission takes place, however high the intensity of the incident radiation may be.
`text(Explanation:)`
Einstein suggested that when a light beam is incident on a metal surface the free electrons of the metal absorb the entire energy of an incident photon during its collision with it. If this electron gets sufficient energy in this manner to do work against the surface adhesion of the given metallic surface and escape then it leaves the metal and a photoelectron is found. For an electron to escape from a metallic surface by doing work against its attractive force and get out of the force field of the metallic surface, a minimum amount of energy is required to be supplied to electrons. This minimum energy required for an electron to escape from a metallic surface is called the work function ofthe given metal which is characteristic of the material and is hence different for different metals. Work function of a given metal is generally represented by the symbol `phi`.
The minimum frequency of light corresponding to which the energy of a photon is equal to the work function of a given metal is called the Threshold frequency of that metal and the corresponding wavelength is called Threshold wavelength Threshold wavelength.
`hv_0 = `phi` or v_0 = phi /h`
Where `v_0` is called threshold frequency.
`(hc)/(lambda_0) = phi` or `lambda_0 = (hc)/phi`
So, or Where `lamda_0` is called threshold wavelength.
Clearly, when a light beam of frequency less than `v_0` or wavelength greater than `lamda_0` is incident then no photoelectrons can be emitted, no matter how high is the intensity ofthe incident beam. Suppose, a photon transfers energy more than the work function of the given metal then the photoelectron may be ejected with a kinetic energy.
`K_(max) = (hv - phi)`
or less than that because a part or all of the extra energy may be lossedduring several collisions that the electron makes before emission.
If the frequency of the photon is v and threshold frequency for the metal is `v_0` , then
`K_(max) = h (v - v_0)`
If the wavelength of the photon is A. and threshold wavelength forthe metal is `lamda_0` , then
`K_(max) = hc (1/ lamda - 1/ lamda_0)`
`text(Stopping Potential:)`
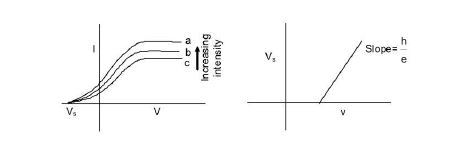
If the polarity of the battery is reversed and the applied potential is gradually increased, the photo current starts decreasing. This is because the electrons are retarded, and most of the elecrons are unable to reach the opposite electrode. It is observed that when the applied retarding potential is increased, the photo current eventually becomes zero. This potential is known as the stopping potential and depends only on the material of the photo cathode and the frequency of light. If `V_s` be the stopping potential, then
`eV_s = hv -phi`
The stopping potential `V_s` depends only on the metal and does not depend on the intensity of incident light. a, b, c -different intensites.
Observation of the experiments on Photo-Electric Effect:
(i) The emission of photoelectrons is instantaneous.
(ii) the number of photoelectrons emitted per second is proportional to the intensity of the incident light.
(iii) The maximum velocity with which electrons emerge is dependent only on the frequency and not on the intensity of the incident light.
(iv) There is always a lower limit of frequency called threshold frequency below which no emission takes place, however high the intensity of the incident radiation may be.
`text(Explanation:)`
Einstein suggested that when a light beam is incident on a metal surface the free electrons of the metal absorb the entire energy of an incident photon during its collision with it. If this electron gets sufficient energy in this manner to do work against the surface adhesion of the given metallic surface and escape then it leaves the metal and a photoelectron is found. For an electron to escape from a metallic surface by doing work against its attractive force and get out of the force field of the metallic surface, a minimum amount of energy is required to be supplied to electrons. This minimum energy required for an electron to escape from a metallic surface is called the work function ofthe given metal which is characteristic of the material and is hence different for different metals. Work function of a given metal is generally represented by the symbol `phi`.
The minimum frequency of light corresponding to which the energy of a photon is equal to the work function of a given metal is called the Threshold frequency of that metal and the corresponding wavelength is called Threshold wavelength Threshold wavelength.
`hv_0 = `phi` or v_0 = phi /h`
Where `v_0` is called threshold frequency.
`(hc)/(lambda_0) = phi` or `lambda_0 = (hc)/phi`
So, or Where `lamda_0` is called threshold wavelength.
Clearly, when a light beam of frequency less than `v_0` or wavelength greater than `lamda_0` is incident then no photoelectrons can be emitted, no matter how high is the intensity ofthe incident beam. Suppose, a photon transfers energy more than the work function of the given metal then the photoelectron may be ejected with a kinetic energy.
`K_(max) = (hv - phi)`
or less than that because a part or all of the extra energy may be lossedduring several collisions that the electron makes before emission.
If the frequency of the photon is v and threshold frequency for the metal is `v_0` , then
`K_(max) = h (v - v_0)`
If the wavelength of the photon is A. and threshold wavelength forthe metal is `lamda_0` , then
`K_(max) = hc (1/ lamda - 1/ lamda_0)`
`text(Stopping Potential:)`
If the polarity of the battery is reversed and the applied potential is gradually increased, the photo current starts decreasing. This is because the electrons are retarded, and most of the elecrons are unable to reach the opposite electrode. It is observed that when the applied retarding potential is increased, the photo current eventually becomes zero. This potential is known as the stopping potential and depends only on the material of the photo cathode and the frequency of light. If `V_s` be the stopping potential, then
`eV_s = hv -phi`
The stopping potential `V_s` depends only on the metal and does not depend on the intensity of incident light. a, b, c -different intensites.