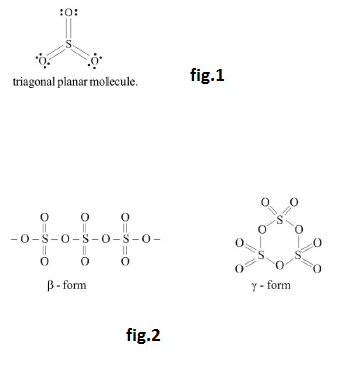
Sulphur di-oxide (`SO_2`) :

See fig.
`ast` `pi` bond for nuclei `d` `pi` - `p` `pi` bond.
`ast` V-shape or trigonal planar.
(ii) `text(Bleaching Property :)` `SO_2` acts as a bleaching agent in following two ways.
(a) In the presence of `H_2O`, it is oxidised with the liberation of nascent hydrogen which reduces the colouring matter to colourless.
`SO_2 + 2H_2O -> H_2SO_4 + 2[H]`
Colouring matter `+ 2[H] ⇋` Colourless compound.
(b) `undersettext(Coloured)(X - OH) + SO_2 -> undersettext(Colourless)(X - HSO_3)`
(iii) `text(Preparations :)`
(a) `text(Lab Method :)` By heating copper turnings with conc. `H_2SO_4`.
`Cu + 2H_2SO_4 text((conc.)) -> CuSO_4 + SO_2 + 2H_2O`
(b) By roasting `ZnS` or iron pyrites.
`2ZnS + 3O_2 -> 2ZnO + 2SO_2`
`4FeS_2 + HO_2 -> 2FeO_3 + 8SO_2`.
(c) Reaction of anhydride with coke :
`2CaSO_4 + C-> 2CaO +CO_2 + 2SO_3`.
(iv) `text(Physical Properties :)`
(a) It is pungent smelling suffocating gas
(b) It is soluble in `H_2O`
`ast` `pi` bond for nuclei `d` `pi` - `p` `pi` bond.
`ast` V-shape or trigonal planar.
(ii) `text(Bleaching Property :)` `SO_2` acts as a bleaching agent in following two ways.
(a) In the presence of `H_2O`, it is oxidised with the liberation of nascent hydrogen which reduces the colouring matter to colourless.
`SO_2 + 2H_2O -> H_2SO_4 + 2[H]`
Colouring matter `+ 2[H] ⇋` Colourless compound.
(b) `undersettext(Coloured)(X - OH) + SO_2 -> undersettext(Colourless)(X - HSO_3)`
(iii) `text(Preparations :)`
(a) `text(Lab Method :)` By heating copper turnings with conc. `H_2SO_4`.
`Cu + 2H_2SO_4 text((conc.)) -> CuSO_4 + SO_2 + 2H_2O`
(b) By roasting `ZnS` or iron pyrites.
`2ZnS + 3O_2 -> 2ZnO + 2SO_2`
`4FeS_2 + HO_2 -> 2FeO_3 + 8SO_2`.
(c) Reaction of anhydride with coke :
`2CaSO_4 + C-> 2CaO +CO_2 + 2SO_3`.
(iv) `text(Physical Properties :)`
(a) It is pungent smelling suffocating gas
(b) It is soluble in `H_2O`