General Properties :
`ast` HALO : Sea Salts: Generally they are called halogen because they are sea salts forming elements.
`ast` Important point : `At` (astatine) is a radioactive element.
`ast` General Properties :
`ast` State and Nature :
(a) `F` and `Cl` are Gas (b) Br : Liquid
(c) `I`, `At` is solid (d) All are non metallic
(e) Metallic character down the group `(F -> I)` non metallic character decreases
(f) `I` has metallic lustre on heating and it shows sublimation property.
`ast` `text(Atomic Radii, Ionic Radii, Boiling point and Melting point, Density :)` All these character increases down the group
`ast` `text(Ionisation potential and Electronegativity :)` Down the group atomic size increases so `IP` and `EN` decreases down the group
`ast` `text(Electron Affinity (E.A.):)` `Cl > F > Br > I`. Generally down the group electron affinity decreases but the electron affinity of `Cl` is
more than `F` because due to the small size and high electron density of `F` the incoming electron is not easily enters as compared to `Cl` because it's size not as small as size of `F` and not large as `Br` and `I`.
`text(Colour Properties :)`
`F` : Pale yellow. `Cl` : Greenish yellow `Br` : Red `I` : Voilet purple.
Outermost electronic configuration is : `ns^2 np^5` due to the presence of unpaired electron they absorb visible light and reflect complementary light and exhibit colour.
`F` absorbs violet colour light and appears yellow. It will absorb yellow coloured light and appears violet.
(d) Astatine being stable could have absorbed orange or red light and would have exhibited indigo or bluish colour.
`ast` `text(Valency and Oxidation state :)`
(a) `ns^2np^5`
(b) valency = 1
If Halogens combines with more `E.N.` elements then `O.S. = +1`
If Halogens combines with more `E.N.` elements then `O.S. = +1`
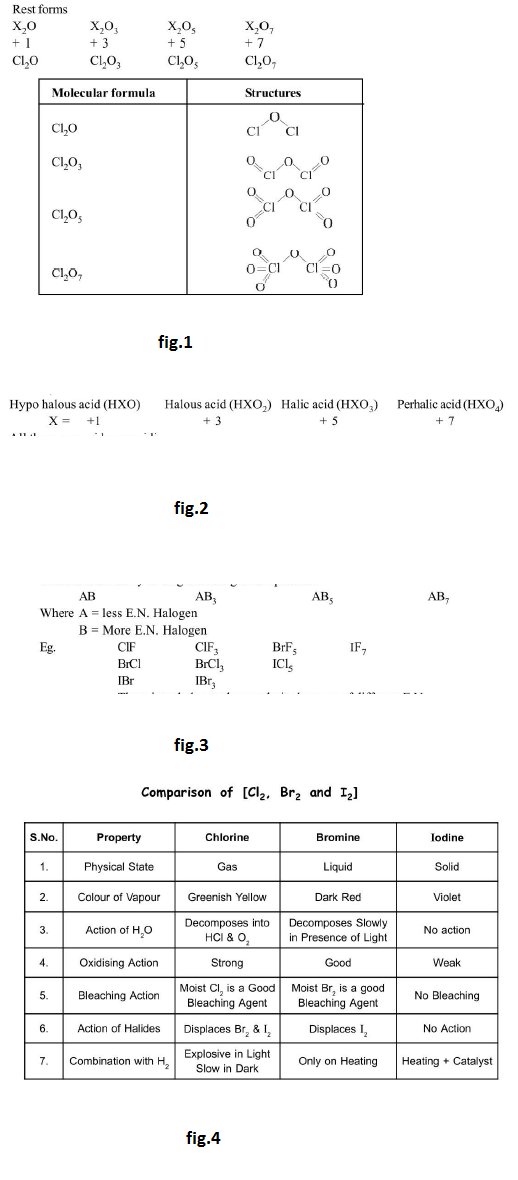
(c) For `Cl`, Ground State : See fig.1.
`ast` `text(Bond Energy :)`
Bond Energy :
`F-F` bond dissociation energy is less than that of `Cl - Cl` and `Br - Br`. It is due to larger inter electronic (electron- electron) repulsion between the non bonding electrons in the `2p` orbitals of fluorine atom, then these in the `3p` orbitals of chlorine atoms.
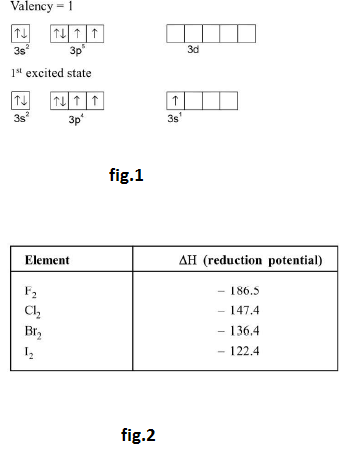
`star` `text(Oxidising Power :)` The electron affinity, or tendency to gain electrons reaches a maximum at chlorine. Oxidation may be regarded as the removal of electron so that an oxidising agent gains electrons. Thus the halogens act as oxidi zing agents. The strength of an oxidising agent (i.e. oxidation potential) depends upon several energy terms and represented by following diagram.
`X_2 overset(DeltaH)-> 2X underset[(Delta H)_(eg)] overset[(+)2e^(-)]-> 2X^(-) overset(Delta H)-> `hydrated ion
`Delta H` (reduction potential) `= Delta H` (dissociation energy) `+ Delta H` (electron gain enthalpy) `+ Delta H`
See fig.2.
Thus oxidising powers decrease on descending in group `VII`. Fluorine is so strong oxidising agent that H oxidizes water to oxygen. The oxidation of `H_2O` by `Cl_2` is thermodynamically possible but since the energy of activation is high this reaction does not occur.
`F_2 + H_2O -> 2H^(+) + 2F^(-) +1/2 O_2`
`Cl_2 +H_2O -> HCl + HOCl`
Iodine is even weaker oxidising agent and the free energy change indicate that energy would have to be supplied to make it oxidise water.
`ast` Important point : `At` (astatine) is a radioactive element.
`ast` General Properties :
`ast` State and Nature :
(a) `F` and `Cl` are Gas (b) Br : Liquid
(c) `I`, `At` is solid (d) All are non metallic
(e) Metallic character down the group `(F -> I)` non metallic character decreases
(f) `I` has metallic lustre on heating and it shows sublimation property.
`ast` `text(Atomic Radii, Ionic Radii, Boiling point and Melting point, Density :)` All these character increases down the group
`ast` `text(Ionisation potential and Electronegativity :)` Down the group atomic size increases so `IP` and `EN` decreases down the group
`ast` `text(Electron Affinity (E.A.):)` `Cl > F > Br > I`. Generally down the group electron affinity decreases but the electron affinity of `Cl` is
more than `F` because due to the small size and high electron density of `F` the incoming electron is not easily enters as compared to `Cl` because it's size not as small as size of `F` and not large as `Br` and `I`.
`text(Colour Properties :)`
`F` : Pale yellow. `Cl` : Greenish yellow `Br` : Red `I` : Voilet purple.
Outermost electronic configuration is : `ns^2 np^5` due to the presence of unpaired electron they absorb visible light and reflect complementary light and exhibit colour.
`F` absorbs violet colour light and appears yellow. It will absorb yellow coloured light and appears violet.
(d) Astatine being stable could have absorbed orange or red light and would have exhibited indigo or bluish colour.
`ast` `text(Valency and Oxidation state :)`
(a) `ns^2np^5`
(b) valency = 1
If Halogens combines with more `E.N.` elements then `O.S. = +1`
If Halogens combines with more `E.N.` elements then `O.S. = +1`
(c) For `Cl`, Ground State : See fig.1.
`ast` `text(Bond Energy :)`
Bond Energy :
`F-F` bond dissociation energy is less than that of `Cl - Cl` and `Br - Br`. It is due to larger inter electronic (electron- electron) repulsion between the non bonding electrons in the `2p` orbitals of fluorine atom, then these in the `3p` orbitals of chlorine atoms.
`star` `text(Oxidising Power :)` The electron affinity, or tendency to gain electrons reaches a maximum at chlorine. Oxidation may be regarded as the removal of electron so that an oxidising agent gains electrons. Thus the halogens act as oxidi zing agents. The strength of an oxidising agent (i.e. oxidation potential) depends upon several energy terms and represented by following diagram.
`X_2 overset(DeltaH)-> 2X underset[(Delta H)_(eg)] overset[(+)2e^(-)]-> 2X^(-) overset(Delta H)-> `hydrated ion
`Delta H` (reduction potential) `= Delta H` (dissociation energy) `+ Delta H` (electron gain enthalpy) `+ Delta H`
See fig.2.
Thus oxidising powers decrease on descending in group `VII`. Fluorine is so strong oxidising agent that H oxidizes water to oxygen. The oxidation of `H_2O` by `Cl_2` is thermodynamically possible but since the energy of activation is high this reaction does not occur.
`F_2 + H_2O -> 2H^(+) + 2F^(-) +1/2 O_2`
`Cl_2 +H_2O -> HCl + HOCl`
Iodine is even weaker oxidising agent and the free energy change indicate that energy would have to be supplied to make it oxidise water.