Estimation of Carbon and Hydrogen :
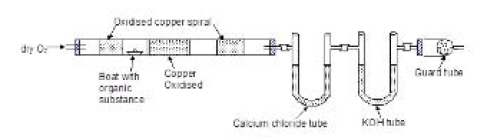
`text(Liebig's Combustion Method)` : A known mass of the organic compound is heated in a current of dry oxygen (free from `CO_2`) in the presence of cupric oxide till all the carbon is oxidised to carbon dioxide and all the oxygen is oxidised to water.
`C_x H_y + (x + y/4) O_2 -> x CO_2 + y/2 H_2O`
Water is absorbed in a previously weighed `U`-tube containing anhydrous calcium chloride or anhydrous magnesium perchlorate. Carbon dioxide is absorbed in a previously weighed `U`-tube containing a strong solution of potassium hydroxide or ascarite (`NaOH` + `CaO`). The weights of carbon dioxide and water thus formed are determined and the amounts of carbon and hydrogen in the organic compound can be calculated as
Moles of `CO_2` formed `= (text(Mass of)quad CO_2 quad text(formed))/44 =` Moles of `C` in `CO_2`
= Moles of `C` in organic compound
Mass of carbon in organic compound `= (text(Mass of)quad CO_2 quad text(formed))/44 xx 12`
Percentage of carbon in organic compound `= 12/44 xx (text(Mass of)quad CO_2 quad text(formed))/(text(Mass of organic compound)) xx 100`
Moles of `H_2O` formed `= (text(Mass of)quad H_2O quadtext(formed))/18`
Moles of `H` in `H_2O` `= (text(Mass of) quadH_2O quadtext(formed))/18 xx 2 =` Moles of `H` in the organic compound.
Mass of hydrogen in the organic compound `= (text(Mass of)quad H_2O quadtext(formed) xx 2 xx 1)/18`
Percentage of hydrogen in organic compound `= 2/18 xx (text(Mass of)quad H_2O quadtext(formed))/text(Mass of organic compound) xx 100`
`C_x H_y + (x + y/4) O_2 -> x CO_2 + y/2 H_2O`
Water is absorbed in a previously weighed `U`-tube containing anhydrous calcium chloride or anhydrous magnesium perchlorate. Carbon dioxide is absorbed in a previously weighed `U`-tube containing a strong solution of potassium hydroxide or ascarite (`NaOH` + `CaO`). The weights of carbon dioxide and water thus formed are determined and the amounts of carbon and hydrogen in the organic compound can be calculated as
Moles of `CO_2` formed `= (text(Mass of)quad CO_2 quad text(formed))/44 =` Moles of `C` in `CO_2`
= Moles of `C` in organic compound
Mass of carbon in organic compound `= (text(Mass of)quad CO_2 quad text(formed))/44 xx 12`
Percentage of carbon in organic compound `= 12/44 xx (text(Mass of)quad CO_2 quad text(formed))/(text(Mass of organic compound)) xx 100`
Moles of `H_2O` formed `= (text(Mass of)quad H_2O quadtext(formed))/18`
Moles of `H` in `H_2O` `= (text(Mass of) quadH_2O quadtext(formed))/18 xx 2 =` Moles of `H` in the organic compound.
Mass of hydrogen in the organic compound `= (text(Mass of)quad H_2O quadtext(formed) xx 2 xx 1)/18`
Percentage of hydrogen in organic compound `= 2/18 xx (text(Mass of)quad H_2O quadtext(formed))/text(Mass of organic compound) xx 100`