Estimation of Halogen :
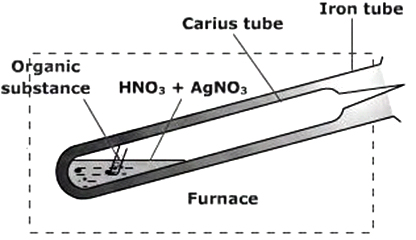
`text(Carius Method)` : In this method, a known mass of the organic substance is heated with fuming nitric acid in the presence of silver nitrate in a special sealed tube known as Carius tube. Carbon, hydrogen or sulphur present in the compound will be oxidised to `CO_2`, `H_2O` and `H_2SO_4` respectively. The halogen forms a precipitate of silver halide (`AgX`). The precipitate is filtered, washed, dried and weighed.
`C + 2O overset(HNO_3)-> CO_2`
`2H +O overset(HNO_3)-> H_2O`
`S + H_2O +3O overset(HNO_3)-> H_2SO_4`
`X +AgNO_3 -> H_2SO_4`
`underset(halogen)X + AgNO_3 -> AgX`
The Carius tube is a long narrow tube closed at one end and (during the experiment) sealed at the other end. A weighed quantity of the organic substance is taken in the tube with fuming nitric acid and silver nitrate, then the open end is sealed and the tube is heated in a furnace. The organic compound decomposes and silver halide is formed. At the end of the experiment, the tube is cooled, the sealed end broken and the contents are transferred to a filter. The precipitated silver halide is filtered, washed, dried and weighed.
Moles of `AgX = (text(Weight of)quad AgX)/(text(Molar mass of)quad AgX) =` Moles of halogen in `AgX`
= Moles of halogen in organic compound
Mass of halogen in organic compound `= ((text(Weight of)quad AgX) xx text(atomic mass of halogen))/(text(molar mass of)quad AgX)`
Percentage of halogen
`= (text(atomic mass of halogen) xx text(w.t of)quad AgX)/(108-text(atomic mass of halogen) xx text(wt. of organic compound) ) xx 100`
`C + 2O overset(HNO_3)-> CO_2`
`2H +O overset(HNO_3)-> H_2O`
`S + H_2O +3O overset(HNO_3)-> H_2SO_4`
`X +AgNO_3 -> H_2SO_4`
`underset(halogen)X + AgNO_3 -> AgX`
The Carius tube is a long narrow tube closed at one end and (during the experiment) sealed at the other end. A weighed quantity of the organic substance is taken in the tube with fuming nitric acid and silver nitrate, then the open end is sealed and the tube is heated in a furnace. The organic compound decomposes and silver halide is formed. At the end of the experiment, the tube is cooled, the sealed end broken and the contents are transferred to a filter. The precipitated silver halide is filtered, washed, dried and weighed.
Moles of `AgX = (text(Weight of)quad AgX)/(text(Molar mass of)quad AgX) =` Moles of halogen in `AgX`
= Moles of halogen in organic compound
Mass of halogen in organic compound `= ((text(Weight of)quad AgX) xx text(atomic mass of halogen))/(text(molar mass of)quad AgX)`
Percentage of halogen
`= (text(atomic mass of halogen) xx text(w.t of)quad AgX)/(108-text(atomic mass of halogen) xx text(wt. of organic compound) ) xx 100`