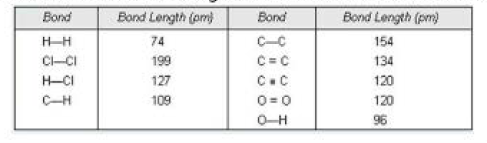
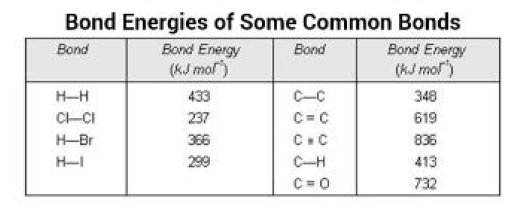
Bond Energy :
It has already been pointed out that the formation of a bond occurs as a result of decrease of energy. Therefore, same amount of energy is required to break the bond between the two atoms. For example, the energy released during the formation of bonds between the gaseous hydrogen atoms to form one mole of hydrogen moleculs is `433` `kJ` `mol^(-1)`. This energy involved in making or breaking of bonds is referred to as bond energy. Thus, bond energy may be defined as the amount of energy required to break one mole of bonds of same kind so as to separate the bonded atoms in the gaseous state.
The magnitude of bond energy reflects the strength of the bond. Its magnitude depends upon the following factors :
(i) `text(Size of the participating atoms)` : Larger the size of the atoms involved in bond formation, lesser is the extent of overlapping and consequently, smaller is the value of bond energy. For example, bond energy of `Cl-Cl` bond is `237` `kJ` `mol^(-1)` whereas that of `H-H` bond is `433` `kJ` `mol^(-1)`.
(ii) `text(Multiplicity of bonds)` : The magnitude of bond energy increases with the multiplicity of bonds even though the atoms involved in the bond formation are same. It is because of the fact that with the multiplicity of bonds the number of shared electrons between the atoms increases. As a result, the attractive force between nuclei and electrons also increases and consequently, the magnitude of bond energy increases. For example, bond energy of `C-C` bond is `348` `kJ` `mol^(-1)` but that of `C=C` bond is `619` `kJ` `mol^(-1)`. The average bond energies of some bonds are given in Table below.
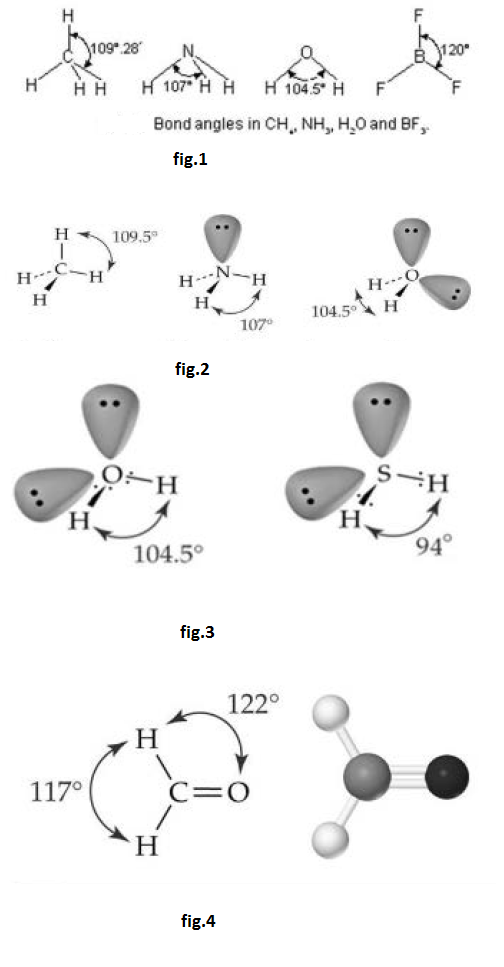
(iii) `text(Number of lone pairs of electrons)` : Greater the number of lone pair of electrons present on the bonded atoms, greater is the repulsive interactions between them and smaller is the bond energy.
The magnitude of bond energy reflects the strength of the bond. Its magnitude depends upon the following factors :
(i) `text(Size of the participating atoms)` : Larger the size of the atoms involved in bond formation, lesser is the extent of overlapping and consequently, smaller is the value of bond energy. For example, bond energy of `Cl-Cl` bond is `237` `kJ` `mol^(-1)` whereas that of `H-H` bond is `433` `kJ` `mol^(-1)`.
(ii) `text(Multiplicity of bonds)` : The magnitude of bond energy increases with the multiplicity of bonds even though the atoms involved in the bond formation are same. It is because of the fact that with the multiplicity of bonds the number of shared electrons between the atoms increases. As a result, the attractive force between nuclei and electrons also increases and consequently, the magnitude of bond energy increases. For example, bond energy of `C-C` bond is `348` `kJ` `mol^(-1)` but that of `C=C` bond is `619` `kJ` `mol^(-1)`. The average bond energies of some bonds are given in Table below.
(iii) `text(Number of lone pairs of electrons)` : Greater the number of lone pair of electrons present on the bonded atoms, greater is the repulsive interactions between them and smaller is the bond energy.