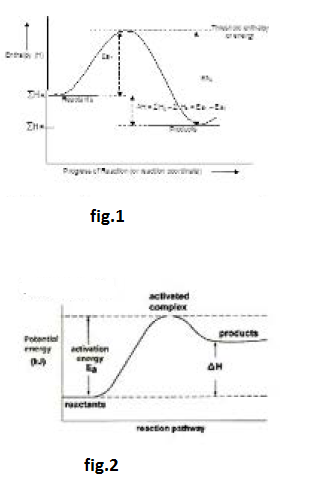
Arrhenius Equation :
The variation equilibrium constant of a reaction with temperature is described by Van't Hoff equation of thermodynamics which is as
follows :
`(dlnK_p)/(dT)=(Delta H)/(RT^2)`
If `k_1` and `k_2` be the rate constants of `FR` (forward reaction) and `BR` (backward reaction), respectively then `K_p = k_1//k_2`. Further, `D H` `= E_(a_1)- E_(a_2)`. Putting these in the above equation we get
`(dlnk_1)/(dT)-(dlnk_2)/(dT)=E_(a_1)/(RT^2)-E_(a_2)/(RT^2)`
Splitting into two parts `(d ln k_1)/(dT) =(E_(a_1))/(RT^2)+k` (For FR)
`(d ln k_2)/(dT) =E_(a_2)/(RT^2) +k` (For BR) where `k` is constant
Arrhenius sets `k` equal to zero and without specifying `FR` and `BR`, he gave the following equation called Arrhenius equation.
`(dlnk)/(dT)=E_a/(RT^2)`...............(4)
From this equation it is evident that rate of change of logarithm of rate constant with temperature depends upon the magnitude of energy of activation of the reaction. Higher the `E_(a_1)` smaller the rate of change of logarithm of rate constant with temperature. That is, rate of the reaction with low `E_a` increases slowly with temperature while rate of the reaction with high `E_a` increases rapidly with temperature. It is also evident that rate of increase of logarithm of rate constant will go on decreasing with increase of temperature.
Integrating Equation `4` assuming `E_a` to be constant we get,
`lnk=-(E_a)/(RT)+ln A`.............(5)
or `ln (k/A)=-(E_a)/(RT)` or `k=Ae^((-E_a)// (RT))`.........(6)
Equation (6) is integrated form of Arrhenius equation. The constant `A` called pre-exponential factor or the frequency factor since it is
somewhat related with collision frequency. It is a constant for a given reaction. From Equation (6) it is evident that as `T ->oo` ,`k->A`. Thus, the constant `A` is the rate constant of reaction at infinity temperature. The rate constant goes on increasing with temperature.
So, when `T` approaches infinity, `k` will be maximum. That is to say, `A` is the maximum rate constant of a reaction. It is also to be noted that the exponential term i.e. `e^(-E_a//RT)` measures the fraction of total number of molecules in the activated state or fraction of the total number of effective collisions. If `n_(E_a)` and `n` be the number of molecules of reactant in the activated state and the total number of molecules of the reactant present in the reaction vessel respectively, then
`(n_(Ea))/n=e^(-E_a//RT)`
Equation (5) may also be put as
`log k = (-(E_a)/(2.303 R)) 1/T +log A` ..............(7)
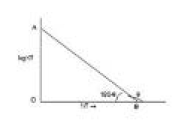
Since `(E_a)/(2.303R)` and `logA` both are constants for a given reaction. So, from equation (7) it is evident that a plot of log `k` vs. `1/T` will be a straight line of the slope equal to `(-E_a)/(2.303 R)` and intercept equal to `logA` as shown in fig.
`(-E_a)/(2.303 R)-tantheta =-tan (180-theta)=-(OA)/(OB)`
`E_a=(OA)/(OB) xx 2.303 R`
`log A=OA`
Thus, from this plot `E_a` and `A` both can be determined accurately
If `k_1`(of first order) be the rate constant of a reaction at two different temperature `T_ 1` and `T_ 2` respectively then from equation (7), we may write
`log k_1 = -E_a/(2.303 R) xx 1/(T_1) +log A`
`log k_2= -E_a/(2.303 R) xx 1/T_2 +log A`
Subtracting former from the latter we get
`log (k_2/k_1) =E_a/(2.303 R) (1/T_1 - 1/T_2)`..............(8)
With the help of this equation it is possible to calculate `E_a` of a reaction provided, rate constants of reaction at two different temperatures are known. Alternatively one can calculate rate constant of a reaction at a given temperature provided that rate constant of the reaction at some other temperature and also `E_a` of the reaction is known.
follows :
`(dlnK_p)/(dT)=(Delta H)/(RT^2)`
If `k_1` and `k_2` be the rate constants of `FR` (forward reaction) and `BR` (backward reaction), respectively then `K_p = k_1//k_2`. Further, `D H` `= E_(a_1)- E_(a_2)`. Putting these in the above equation we get
`(dlnk_1)/(dT)-(dlnk_2)/(dT)=E_(a_1)/(RT^2)-E_(a_2)/(RT^2)`
Splitting into two parts `(d ln k_1)/(dT) =(E_(a_1))/(RT^2)+k` (For FR)
`(d ln k_2)/(dT) =E_(a_2)/(RT^2) +k` (For BR) where `k` is constant
Arrhenius sets `k` equal to zero and without specifying `FR` and `BR`, he gave the following equation called Arrhenius equation.
`(dlnk)/(dT)=E_a/(RT^2)`...............(4)
From this equation it is evident that rate of change of logarithm of rate constant with temperature depends upon the magnitude of energy of activation of the reaction. Higher the `E_(a_1)` smaller the rate of change of logarithm of rate constant with temperature. That is, rate of the reaction with low `E_a` increases slowly with temperature while rate of the reaction with high `E_a` increases rapidly with temperature. It is also evident that rate of increase of logarithm of rate constant will go on decreasing with increase of temperature.
Integrating Equation `4` assuming `E_a` to be constant we get,
`lnk=-(E_a)/(RT)+ln A`.............(5)
or `ln (k/A)=-(E_a)/(RT)` or `k=Ae^((-E_a)// (RT))`.........(6)
Equation (6) is integrated form of Arrhenius equation. The constant `A` called pre-exponential factor or the frequency factor since it is
somewhat related with collision frequency. It is a constant for a given reaction. From Equation (6) it is evident that as `T ->oo` ,`k->A`. Thus, the constant `A` is the rate constant of reaction at infinity temperature. The rate constant goes on increasing with temperature.
So, when `T` approaches infinity, `k` will be maximum. That is to say, `A` is the maximum rate constant of a reaction. It is also to be noted that the exponential term i.e. `e^(-E_a//RT)` measures the fraction of total number of molecules in the activated state or fraction of the total number of effective collisions. If `n_(E_a)` and `n` be the number of molecules of reactant in the activated state and the total number of molecules of the reactant present in the reaction vessel respectively, then
`(n_(Ea))/n=e^(-E_a//RT)`
Equation (5) may also be put as
`log k = (-(E_a)/(2.303 R)) 1/T +log A` ..............(7)
Since `(E_a)/(2.303R)` and `logA` both are constants for a given reaction. So, from equation (7) it is evident that a plot of log `k` vs. `1/T` will be a straight line of the slope equal to `(-E_a)/(2.303 R)` and intercept equal to `logA` as shown in fig.
`(-E_a)/(2.303 R)-tantheta =-tan (180-theta)=-(OA)/(OB)`
`E_a=(OA)/(OB) xx 2.303 R`
`log A=OA`
Thus, from this plot `E_a` and `A` both can be determined accurately
If `k_1`(of first order) be the rate constant of a reaction at two different temperature `T_ 1` and `T_ 2` respectively then from equation (7), we may write
`log k_1 = -E_a/(2.303 R) xx 1/(T_1) +log A`
`log k_2= -E_a/(2.303 R) xx 1/T_2 +log A`
Subtracting former from the latter we get
`log (k_2/k_1) =E_a/(2.303 R) (1/T_1 - 1/T_2)`..............(8)
With the help of this equation it is possible to calculate `E_a` of a reaction provided, rate constants of reaction at two different temperatures are known. Alternatively one can calculate rate constant of a reaction at a given temperature provided that rate constant of the reaction at some other temperature and also `E_a` of the reaction is known.