Intermixing of Orbital :
The `2s` and `2p` energy levels of `O` and `F` are very far apart. The combination of the `2s` orbitals from the two atoms form a sigma bonding and sigma anti bonding orbitals in a way very similar to the case of the hydrogen molecules, because the `2p` orbitals have little to do with the `2s` orbitals.
On the other hand, the three `2p` orbitals of each `O` (or `F`) atom can form one sigma and two pi bonds and their corresponding antibonding molecular orbitals. The interaction of the `2p` orbitals for the sigma bond is stronger, and the levels of sigma and anti sigma
bonds are farther apart than those of pi and anti pi bonds.
See fig.
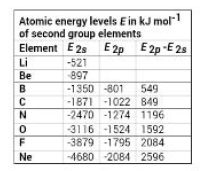
Recently, the study of the energies of electrons in molecules revealled that the relative energy levels of molecular orbitals of `Li_2` to `N_2` are different from those of `O_2` and `F_2.` The explanation for the difference comes from the consideration of hybrid atomic orbitals. Because the `2s` energy levels and `2p` energy levels for `Li` to `N` are relatively close, the `2s` orbitals are influenced by the `2p` orbitals. This influence makes the bonding orbitals stronger than, and the antibonding orbitals weaker than those formed by pure `2s` orbitals. This process is called `sp` mixing
Due to `s p` mixing, the `s_(2p)` orbital is weakened, and the `s_(2p)^(ast)` is also affected. These effects cause the relative order to change.
On the other hand, the three `2p` orbitals of each `O` (or `F`) atom can form one sigma and two pi bonds and their corresponding antibonding molecular orbitals. The interaction of the `2p` orbitals for the sigma bond is stronger, and the levels of sigma and anti sigma
bonds are farther apart than those of pi and anti pi bonds.
See fig.
Recently, the study of the energies of electrons in molecules revealled that the relative energy levels of molecular orbitals of `Li_2` to `N_2` are different from those of `O_2` and `F_2.` The explanation for the difference comes from the consideration of hybrid atomic orbitals. Because the `2s` energy levels and `2p` energy levels for `Li` to `N` are relatively close, the `2s` orbitals are influenced by the `2p` orbitals. This influence makes the bonding orbitals stronger than, and the antibonding orbitals weaker than those formed by pure `2s` orbitals. This process is called `sp` mixing
Due to `s p` mixing, the `s_(2p)` orbital is weakened, and the `s_(2p)^(ast)` is also affected. These effects cause the relative order to change.