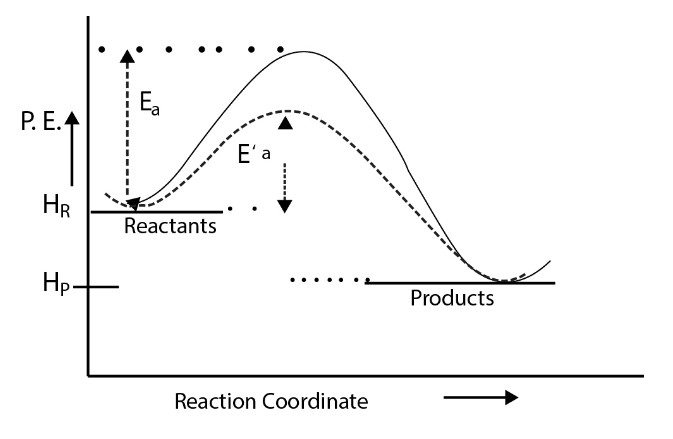
Reaction Mechanism and Rate Determining Step :
To understand what is order of reaction, based on mechanism consider the reaction :
`2NO(g) + 2H_2(g) -> N_2(g) + 2H_2O(g)`
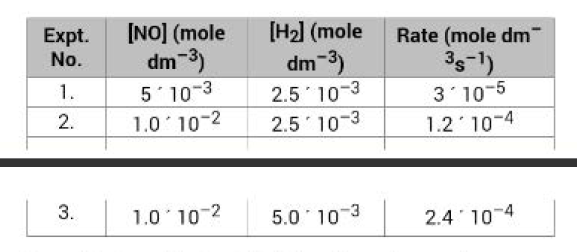
Kinetic experiment carried out at `1100` `K` upon this reaction has shown following rate data : See Table.
From the Expt. No.1 and 2, it is evident that rate increases `4` fold when concentration of `NO` is doubled keeping the concentration of `H_2` constant i.e.
Rate `prop [NO]^2`, when `[H_2]` is constant.
Again from Expt. No.2 and 3, it is evident that when concentration of `H_2` is doubled keeping the concentration of `NO` constant, the rate is just doubled i.e.
Rate `prop [H_2]`, when `[NO]` is constant.
From Expt. (1) and Expt. (3), the rate increases `8`-fold when concentrations of both `NO` and `H_2` are doubled simultaneously i.e.
Rate `prop [NO]^2 [H_2]`
This is the rate-law of reaction as observed experimentally. In the rate law, the power of nitric oxide concentration is `2` while that of hydrogen concentration is `1`. So, order of reaction w.r.t. `NO` is `2` and that w.r.t. `H_2` is `1` and overall order is `2 + 1` i.e. `3`.
`2NO(g) + 2H_2(g) -> N_2(g) + 2H_2O(g)`
Kinetic experiment carried out at `1100` `K` upon this reaction has shown following rate data : See Table.
From the Expt. No.1 and 2, it is evident that rate increases `4` fold when concentration of `NO` is doubled keeping the concentration of `H_2` constant i.e.
Rate `prop [NO]^2`, when `[H_2]` is constant.
Again from Expt. No.2 and 3, it is evident that when concentration of `H_2` is doubled keeping the concentration of `NO` constant, the rate is just doubled i.e.
Rate `prop [H_2]`, when `[NO]` is constant.
From Expt. (1) and Expt. (3), the rate increases `8`-fold when concentrations of both `NO` and `H_2` are doubled simultaneously i.e.
Rate `prop [NO]^2 [H_2]`
This is the rate-law of reaction as observed experimentally. In the rate law, the power of nitric oxide concentration is `2` while that of hydrogen concentration is `1`. So, order of reaction w.r.t. `NO` is `2` and that w.r.t. `H_2` is `1` and overall order is `2 + 1` i.e. `3`.