Formation of Molecular Orbitals :
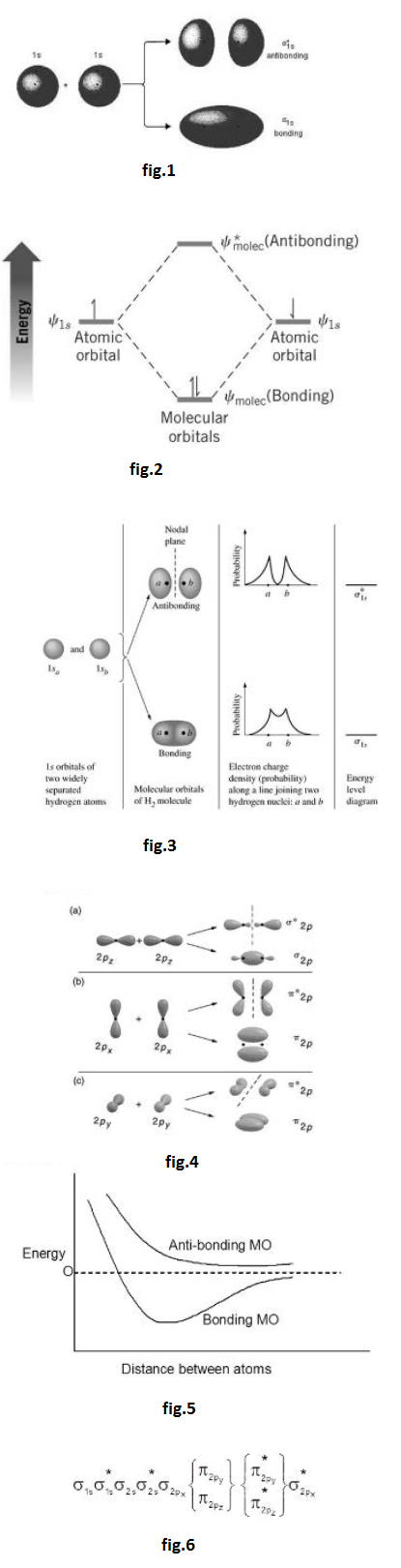
Molecular orbitals are obtained by combining the atomic orbitals of the atoms in the molecule. Consider the `H_2` molecule, for example. One of the molecular orbitals in this molecule is constructed by adding the mathematical functions for the two `1s` atomic orbitals that come together to form this molecule. Another orbital is formed by subtracting one of these functions from the other, as shown in the fig.1.
One of these orbitals is called `text(a bonding molecular orbital)` because electrons in this orbital spend most of their time in the region directly between the two nuclei. It is called a `text(sigma)` (`(sigma)`) molecular orbital because it looks like an `s` orbital when viewed along the `H-H` bond. Electrons placed in the other orbital, spend most of their time away from the region between the two nuclei. This orbital is therefore an `text(antibonding)`, or sigma star `(sigma^(sast)`), molecular orbital. See fig.2.
The `sigma` bonding molecular orbital concentrates electrons in the region directly between the two nuclei. Placing an electron in this orbital therefore stabilizes the `H_2` molecule. Since the `sigma^(ast)` anti-bonding molecular orbital forces the electron to spend most of its time away from the area between the nuclei, placing an electron in this orbital makes the molecule less stable.
Electrons are added to molecular orbitals, one at a time, starting with the lowest energy molecular orbital. The two electrons associated with a pair of hydrogen atoms are placed in the lowest energy, or `sigma` bonding, molecular orbital, as shown in the fig.3. This diagram suggests that the energy of an `H_2` molecule is lower than that of a pair of isolated atoms. As a result, the `H_2` molecule is more stable than a pair of isolated atoms.
If we arbitrarily define the `z`-axis of the coordinate system for the `O_2` molecule as the axis along which the bond forms, the `2p^2` orbitals on the adjacent atoms will meet head-on to form a `sigma` `2p` bonding and a `sigma` `2p^(ast)` antibonding molecular orbital, as shown in the figure below. See fig.4.
The `2p_x` orbitals on one atom interact with the `2p_x` orbitals on the other to form molecular orbitals that have a different shape. These molecular orbitals are calledpi `(pi)` orbitals because they look like `p`-orbitals when viewed along the bond. Whereas `sigma` and `sigma^(star)` orbitals concentrate the electrons along the axis on which the nuclei of the atoms lie, `pi` and `pi^(ast)` orbitals concentrate the electrons either above or below this axis.
The `2 p_x` atomic orbitals combine to form a `pi_x` bonding molecular orbital and a `pi_x^(ast)` antibonding molecular orbital. The same thing happens when the `2p_y` orbitals interact, only in this case we get a `pi_y` and a `pi_y^(ast)` antibonding molecular orbital. Because there is no difference between the energies of the `2p_x` and `2p_y` atomic orbitals, there is no difference between the energies of the `pi_x` and `pi_y` or the `pi_x^(ast)` and `pi_y^(ast)` molecular orbitals.
The number of molecular orbitals produced must always be equal to the number of atomic orbitals involved. Electron density is increased for the bonding `MO's` in the inter-nuclear region but decreased for the anti-bonding `MO's`, Shielding of the nuclei by increased electron density in bonding `MO's` reduces inter-nuclei repulsion and thus stabilizes the molecule whereas lower electron density even as compared to the individual atom in anti-bonding `MO's` increases the repulsion and destabilizes the system. See fig.5
In denotion of `MO's`, `s` indicates head on overlap and `p` represents side ways overlap of orbitals. In simple homonuclear diatomic molecules the order of MO's based on increasing energy is see fig.6.
Note that the `2p_y` atomic orbitals gives `p` bonding and `p`* anti-bonding `MOs` and the `2p_z` atomic orbital gives `p` bonding and `p^ast` anti-bonding `MOs`. The bonding `p` `2p_y` `MOs` have exactly the same energy and are said to be double degenerate and in a similar way `p^(ast)2p_y` and `p 2p_z` are also doubly degenerate.
This order is true except `B_2`, `C_2` & `N_2`. For them `p 2p_y` and `p 2p_z` are probably lower than `s` `2p_x`
Bond order= `(text(no. of) e^(-) text(occupying bonding MOs) - text(no. of) e^(-) text(occupying anti-bonding MOs))/2`
Atoms or molecules in which the electrons are paired are diamagnetic-repelled by both poles of a magnetic. Those that have one or more unpaired electrons are paramagnetic attracted to a magnetic field. Liquid oxygen is attracted to a magnetic field and can actually bridge the gap between the poles of a horse shoe magnet. The molecular orbital model of `O_2` is therefore superior to the valence-bond model, which cannot explain this property of oxygen.
One of these orbitals is called `text(a bonding molecular orbital)` because electrons in this orbital spend most of their time in the region directly between the two nuclei. It is called a `text(sigma)` (`(sigma)`) molecular orbital because it looks like an `s` orbital when viewed along the `H-H` bond. Electrons placed in the other orbital, spend most of their time away from the region between the two nuclei. This orbital is therefore an `text(antibonding)`, or sigma star `(sigma^(sast)`), molecular orbital. See fig.2.
The `sigma` bonding molecular orbital concentrates electrons in the region directly between the two nuclei. Placing an electron in this orbital therefore stabilizes the `H_2` molecule. Since the `sigma^(ast)` anti-bonding molecular orbital forces the electron to spend most of its time away from the area between the nuclei, placing an electron in this orbital makes the molecule less stable.
Electrons are added to molecular orbitals, one at a time, starting with the lowest energy molecular orbital. The two electrons associated with a pair of hydrogen atoms are placed in the lowest energy, or `sigma` bonding, molecular orbital, as shown in the fig.3. This diagram suggests that the energy of an `H_2` molecule is lower than that of a pair of isolated atoms. As a result, the `H_2` molecule is more stable than a pair of isolated atoms.
If we arbitrarily define the `z`-axis of the coordinate system for the `O_2` molecule as the axis along which the bond forms, the `2p^2` orbitals on the adjacent atoms will meet head-on to form a `sigma` `2p` bonding and a `sigma` `2p^(ast)` antibonding molecular orbital, as shown in the figure below. See fig.4.
The `2p_x` orbitals on one atom interact with the `2p_x` orbitals on the other to form molecular orbitals that have a different shape. These molecular orbitals are calledpi `(pi)` orbitals because they look like `p`-orbitals when viewed along the bond. Whereas `sigma` and `sigma^(star)` orbitals concentrate the electrons along the axis on which the nuclei of the atoms lie, `pi` and `pi^(ast)` orbitals concentrate the electrons either above or below this axis.
The `2 p_x` atomic orbitals combine to form a `pi_x` bonding molecular orbital and a `pi_x^(ast)` antibonding molecular orbital. The same thing happens when the `2p_y` orbitals interact, only in this case we get a `pi_y` and a `pi_y^(ast)` antibonding molecular orbital. Because there is no difference between the energies of the `2p_x` and `2p_y` atomic orbitals, there is no difference between the energies of the `pi_x` and `pi_y` or the `pi_x^(ast)` and `pi_y^(ast)` molecular orbitals.
The number of molecular orbitals produced must always be equal to the number of atomic orbitals involved. Electron density is increased for the bonding `MO's` in the inter-nuclear region but decreased for the anti-bonding `MO's`, Shielding of the nuclei by increased electron density in bonding `MO's` reduces inter-nuclei repulsion and thus stabilizes the molecule whereas lower electron density even as compared to the individual atom in anti-bonding `MO's` increases the repulsion and destabilizes the system. See fig.5
In denotion of `MO's`, `s` indicates head on overlap and `p` represents side ways overlap of orbitals. In simple homonuclear diatomic molecules the order of MO's based on increasing energy is see fig.6.
Note that the `2p_y` atomic orbitals gives `p` bonding and `p`* anti-bonding `MOs` and the `2p_z` atomic orbital gives `p` bonding and `p^ast` anti-bonding `MOs`. The bonding `p` `2p_y` `MOs` have exactly the same energy and are said to be double degenerate and in a similar way `p^(ast)2p_y` and `p 2p_z` are also doubly degenerate.
This order is true except `B_2`, `C_2` & `N_2`. For them `p 2p_y` and `p 2p_z` are probably lower than `s` `2p_x`
Bond order= `(text(no. of) e^(-) text(occupying bonding MOs) - text(no. of) e^(-) text(occupying anti-bonding MOs))/2`
Atoms or molecules in which the electrons are paired are diamagnetic-repelled by both poles of a magnetic. Those that have one or more unpaired electrons are paramagnetic attracted to a magnetic field. Liquid oxygen is attracted to a magnetic field and can actually bridge the gap between the poles of a horse shoe magnet. The molecular orbital model of `O_2` is therefore superior to the valence-bond model, which cannot explain this property of oxygen.