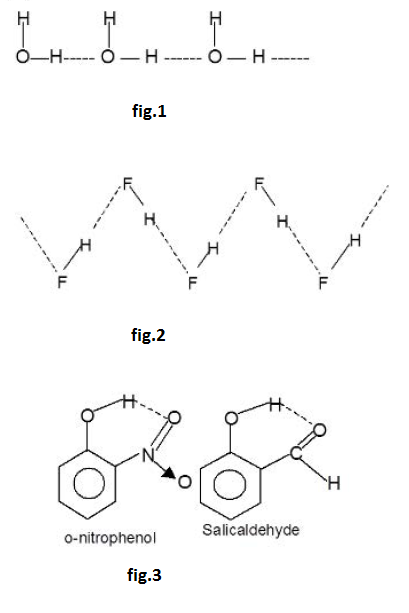
Ahydrogen bond is a type of attractive intermolecular force that exists between two partial electric charge of opposite polarity. Although stronger than most other intermolecular forces, the hydrogen bond is much weaker than both the `text(ionic bond)` and the `text(covalent bond)`. Within macromolecules such as proteins and nucleic acids, it can exist between two parts of the same molecule, and figures as an important constraint on such molecules' overall shape.
As the name "hydrogen bond" implies, one part of the bond involves a `text(hydrogen atom)`. The hydrogen must be attached to a strongly electronegative heteroatom, such as `text(oxygen, nitrogen)` or `text(fluorine)` which is called the hydrogen-bond donor. This electronegative element attracts the electron cloud from around the hydrogen nucleus and, by decentralizing the cloud, leaves the atom with a positive partial charge. Because of the small size of hydrogen relative to other atoms and molecules, the resulting charge,
though only partial, nevertheless represents a large charge density. A hydrogen bond results when this strong positive charge density attracts a `text(lone pair)` of electrons on another heteroatom, which becomes the hydrogen-bond acceptor.
The hydrogen bond is not like a simple attraction between point charges, however. It possesses some degree of orientational preference, and can be shown to have some of the characterist ics of a covalent bond. This covalency tends to be more extreme when acceptors bind hydrogens from more electronegative donors.
In the species `X-H--X` as the electronegativity of `X` increases the strength of hydrogen bond (`H- - - X`) also increases. Thus the order of increases of the and (`F- H- -- - F`) is shown below
`N - H-- --N` < `O - H-- --O` < `F - H- --- F`
[The electronegativity of `N = 3.0`, `O = 3.5`, `F = 4.0` in Pauling scale]
Ahydrogen bond is a type of attractive intermolecular force that exists between two partial electric charge of opposite polarity. Although stronger than most other intermolecular forces, the hydrogen bond is much weaker than both the `text(ionic bond)` and the `text(covalent bond)`. Within macromolecules such as proteins and nucleic acids, it can exist between two parts of the same molecule, and figures as an important constraint on such molecules' overall shape.
As the name "hydrogen bond" implies, one part of the bond involves a `text(hydrogen atom)`. The hydrogen must be attached to a strongly electronegative heteroatom, such as `text(oxygen, nitrogen)` or `text(fluorine)` which is called the hydrogen-bond donor. This electronegative element attracts the electron cloud from around the hydrogen nucleus and, by decentralizing the cloud, leaves the atom with a positive partial charge. Because of the small size of hydrogen relative to other atoms and molecules, the resulting charge,
though only partial, nevertheless represents a large charge density. A hydrogen bond results when this strong positive charge density attracts a `text(lone pair)` of electrons on another heteroatom, which becomes the hydrogen-bond acceptor.
The hydrogen bond is not like a simple attraction between point charges, however. It possesses some degree of orientational preference, and can be shown to have some of the characterist ics of a covalent bond. This covalency tends to be more extreme when acceptors bind hydrogens from more electronegative donors.
In the species `X-H--X` as the electronegativity of `X` increases the strength of hydrogen bond (`H- - - X`) also increases. Thus the order of increases of the and (`F- H- -- - F`) is shown below
`N - H-- --N` < `O - H-- --O` < `F - H- --- F`
[The electronegativity of `N = 3.0`, `O = 3.5`, `F = 4.0` in Pauling scale]