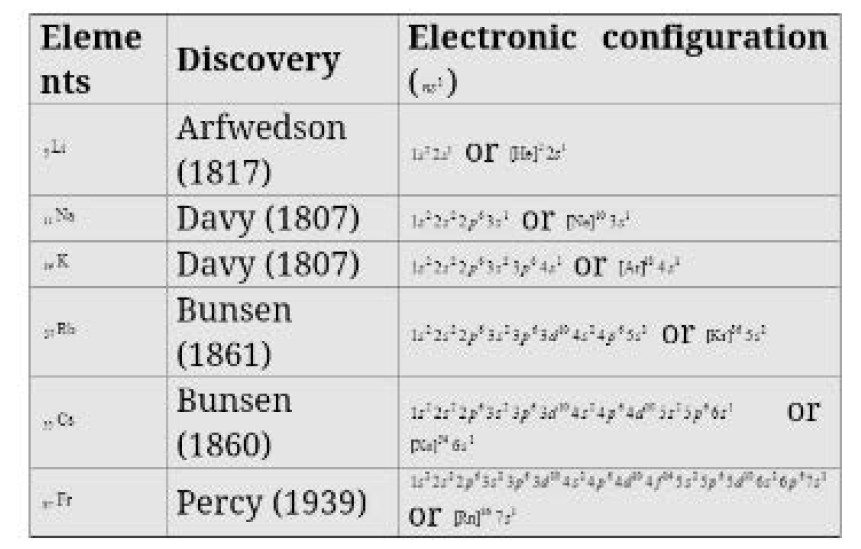
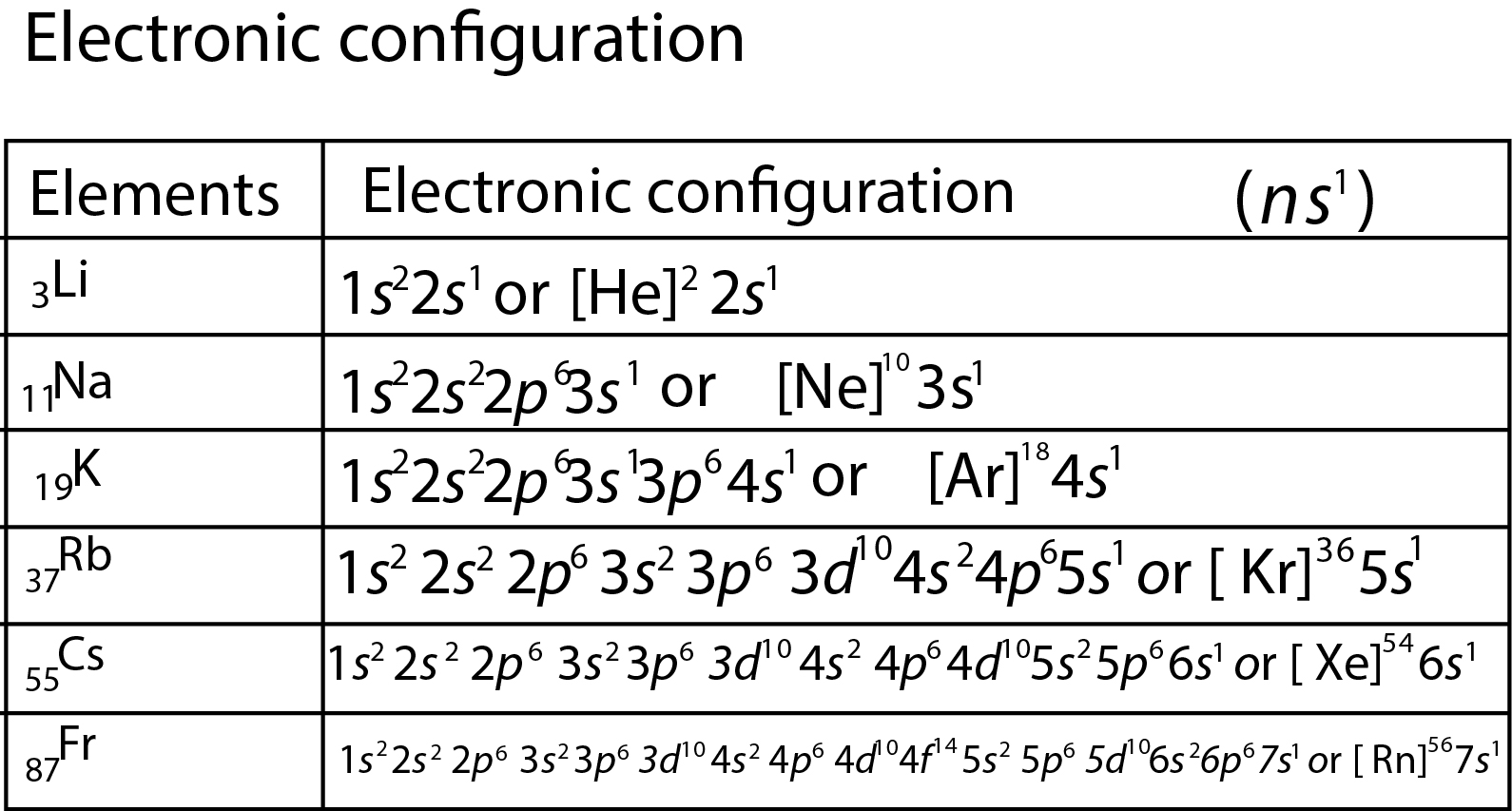
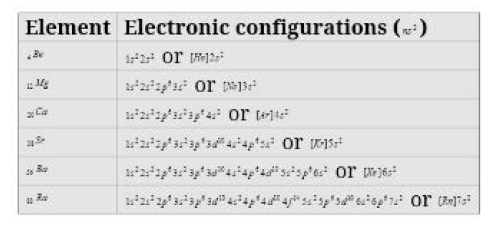
(a) Occurrence : Alkali metals are very reactive and thus found in combined state Some important ores of alkali metals are given ahead.
(i) Lithuinr : Triphylite, Petalite, lepidolite, Spodumene [`LiAl` `( SiO _3)_3`] Amblygonite [ `Li (Al F )PO _4`]
(ii) Sodium : Chile salt petre ( `NaNO_ 3`), Sodium chloride ( `NaCl `), Sodium sulphate (`Na _2 SO_ 4`),
Borax ( `Na_2 B_4O_ 7 10 H _2 O` ), Glauber salt ( `Na _2 SO_4*10H_ 2 O`)
(iii) Potassium : Sylime (`KCl` ), carnallite ( `KCl *MgCl_2 *6 H _2O` )and felspar (`K _2 O * Al_ 2 O_3 *6Si O_2`)
(iv) Rubidium : Lithuim ores Lepidolite, triphylite contains `0.7` to ` 3%` `Rb _2 O`
(v) Caesium : Lepidolite, Pollucite contains `0.2` to `7% Cs_2 O`
(b) Extraction of alkali metals : Alkali metals cannot be extracted by the usual methods for the extraction of metals due to following reasons.
(i) Alkali metals are strong reducing agents, hence cannot be extracted by reduction of their oxides or other compounds.
(ii) Being highly electropositive in nature, it is not possible to apply the method of displacing them from their salt solutions by any other element.
(iii) The aqueous solutions of their salts cannot be used for extraction by electrolytic method because hydrogen ion is discharged at cathode instead of an alkali metal ions as the discharge potentials of alkali metals are high. However, by using `Hg` as cathode, alkali metal can be deposited. The alkali metal readily combines with `Hg` to form an amalgam from which its recovery difficult. The only successful method, therefore, is the electrolysis of their fused salts, usually chlorides. Generally, another metal chloride is added to lower their fussion temperature.
Fused NaCl :
`NaCl overset(fusion)-> Na^+ +Cl^(-) `
Electrolysis of fused salt : Anode : ` 2Cl^(-) ->Cl_2 +2e`
cathode: `2Na^(+) +2 e-> 2 Na`
(c) Alloys Formation :
(i) The alkali metals form alloys among themselves as well as with other metals.
(ii) Alkali metals also get dissolved in mercury to form amalgam with evolution of heat and the amalgamation is highly exothermic.
(d) Formation of oxides and hydroxides :
(i) These are most reactive metals and have strong affinity for `O_2` quickly tranish in air due to the formation of a film of their oxides on the surface. These are, therefore, kept under kerosene or paraffin oil to protect them from air,
`M+O_2-> undersettext(oxide)(M_2O)-> undersettext(peroxide)(M_2O_2)`
(ii) When burnt air (`O_2`), lithium forms lithium oxide (`Li _2 O`) sodium forms sodium peroxide (`Na_2 O_2`) and other alkali metals form super oxide (`MO_2` i.e. `KO _2* RbO _2` or `CsO _2`)
`2Li+1/2 O_2-> Ki_2O; 2Na+O_2-> Na_2O_2; K+O_2-> KO_2`
The reactivity of alkali metals towards oxygen to form different oxides is due to strong positive field around each alkali metal cation. `Li ^+` being smallest, possesses strong positive field and rhus combines with small anion `O^(2-)` to form stable `Li_2O` compound.
The `Na^+` and `K^+` being relatively larger thus exert less strong positive field around them and thus reacts with larger oxygen anion i.e, `O_2^(2-)`to form stable oxides.
The monoxide, peroxides and superoxides have `O^2` and `O_2^(2-)`, `O_2^(1-)` ions respectively. The structures of each are
`[: undersettext(x x)overset(..)O:]^(-2)`, `[: underset(..)overset(..) O - underset(..) overset(..)O:]`, `[:overset(..)O...overset(..)O:]`
The `O_2^(-1)` ion has a three electron covalent bond and has one electron unpaired. It is therefore superoxides are paramagnetic and coloured `KO _2` is light yellow and paramagnetic substance.
(iii) The oxides of alkali metals and metal itself give strongly alkaline solution in water with evolution
of heat
`M+H_2O-> MOH+1/2 H_2 ; Delta=-ve`
`LiO_2 +H_2O ->2LiOH; Delta H=-ve`
`Na_2O_2+2H_2O->2NaOH+H_2O; Delta H=-ve`
`2KO_2+2H_2O->2KOH +H_2O+O_2; Delta H=-ve`
The peroxides and superoxides act as strong oxidising agents due to formation of `H_2O_2`
(iv) The reactivity of alkali metals towards air and water increases from `Li` to `Cs` that is why Jithjum decomposes `H _2O` very slowly at `25^oC` whereas `Na` does so vigorously, K reacts producing a flame and `Rb`, `Cs` do so explosively.
`M+H_2O->MOH+1/2 H_2`
(v) The basic character of oxides and hydroxides of alkali metals increases from `Li` to `Cs`. This is due to the increase in ionic character of alkali metal hydroxides down the group which leads to complete dissociation and leads to increase in concentration of `OH^-` ions.
(e) Hydrides :
(i)These metal combines `H` to give white crystalline ionic hydrides of the general of the formula `MH`.
(ii) The tendency to form their hydrides, basic character and stability decreases from `Li` to `Cs` since the electropositive character decreases from `Cs` to `Li`.
`2M + H_2 -> 2MH`; Reactivity towards `H_2` is `Cs < Rb < K < Na < Li`
(iii) The metal hydrides react with water to give `MOH` & `H_2`; `MH + H_2O -> MOH + H_2`
(iv) The ionic nature of hydrides increases from `Li` to `Cs` because of the fact that hydrogen is present in the these hydrides as `H^-` and the smaller cation will produce more polarisation of anion (according to Fajan rule) and will develop more covalent character.
(v) The electrolysis of fused hydrides give `H_2` at anode. `(NaH_(text(fused))text(Contains)) Na^(+) text(and) H^(-) ` i.e.,
At cathode: `Na^(+) + e -> Na;` At anode: `H^(-) -> 1/2H_2 + e`
(vi) Alkali metals also form hydrides like `NaBH_4,` `Li.A I H_4` which are good reducing agent.
(f) Carbonates and Bicarbonate :
(i) The carbonates `(M_2CO_3)` & bicarbonates `(MHCO_3)` are highly stable to heat, where `M` stands for alkali metals.
(ii) The stability of these salts increases with the increasing electropositive character from Li to Cs. It is therefore Liz `CO_3` decompose on heating, `Li_2CO_3 -> Li_2O + CO_2`
(iii) Bicarbonates are decomposed at relatively low temperature,
`2MHCO_3 overset(300^oC)-> M_2CO_3 +H_2O +CO_2`
(iv) Both carbonates and bicarbonates are soluble in water to give alkaline solution due to hydrolysis of carbonate ions or bicarbonate ions.
(g) Halides :
(i) Alkali metals combine directly with halogens to form ionic halide `M^(+) X^(-)`.
(ii) The ease with which the alkali metals form halides increases from `Li` to `Cs` due to increasing electropositive character from `Li` to `Cs`.
(iii) Lithium halides however have more covalent nature. Smaller is the cation, more is deformation of anion and thus more is covalent nature in compound. Also among lithium halides, lithium iodide has maximum covalent nature because of larger anion which is easily deformed by a cation (The Fajan's rule) Thus covalent character in lithium halides is, `LiI > LiBr > LiCl > LiF`
(iv) These are readily soluble in water. However, lithium fluoride is sparingly soluble. The low solubility of `LiF` is due to higher forces of attractions among smaller `Li^(+)` and smaller `F^(-)` ions (high lattice energy).
(v) Halides having ionic nature have high m.pt. and good conductor of current. The melting points of halides shows the order, `NaF > NaCl > NaBr > NaI`
(vi) Halides of potassium, rubidium and caesium have a property of combining with extra halogen atoms forming polyhalides.
`KI + I_2 -> KI_3`; In `KI_(3(aq))` the ions `K^(+)` and `I_3^(-)` are present.
(h) Solubility in liquid `NH_3` :
(i) These metals dissolve in liquid NH 3 to produce blue coloured solution, which conducts electricity to an appreciable degree.
(ii) With increasing concentration of ammonia, blue colour starts changing to that of metallic copper after which dissolution of alkali metals in `NH_3` ceases.
(iii) The metal atom is converted into ammoniated metal in i.e. `M^(+) (NH_3)` and the electron set free combines with `NH_3` molecule to produce ammonia solvated electron .
`Na +(x +y) -> undersettext(Ammoniated cation){NH_3[Na(NH_3)_x]^+} + undersettext(ammoniated electron){[e(NH_3)]^-}`
(iv) It is the ammoniated electron which is responsible for blue colour, paramagnetic nature and reducing power of alkali metals in ammonia solution. However, the increased conductance nature of these metals in ammonia is due to presence of ammoniated cation and ammonia solvated electron.
(v) The stability of metal-ammonia solution decreases from `Li` to `Cs`.
(vi) The blue solution on standing or on heating slowly liberates hydrogen, `2M + 2NH_3 -> 2MNH_2 + H_2` . Sodamide `(NaNH_2)` is a waxy solid, used in preparation of number of sodium compounds.
(i) Nitrates : Nitrates of alkali metals (`MNO_3`) are soluble in water and decompose on heating. `LiNO_3` decomposes to give `NO_2` and `O_2` and rest all give nitrites and oxygen.
`2MNO_3 -> 2MNO_2 + O_2` (except Li) ; `4 LiNO_3 -> 2Li_2O + 4NO_2 + O_2`
(j) Sulphates :
(i) Alkali metals' sulphate have the formula `M_2SO_4`.
(ii) Except `Li_2SO_4,` rest all are soluble water.
(iii) These sulphates on fusing v.rith carbon form sulphides, `M_2SO_4 + 4C -> M_2S + 4CO`
(iv) The sulphates of alkali metals (except Li) form double salts with the sulphate of the trivalent metals like Fe, AI, Cr etc. The double sulphates crystallize with large munber of water molecules as alum. e.g. `K_2SO_4 . Al_2 (SO_4)_3. 24 H_2O`
(k) Reaction with non-metals :
(i) These have high affinity for non-metals. Except carbon and nitrogen, they directly react with hydrogen, halogens, sulphur, phosphorus etc. to form corresponding compounds on heating.
`2Na + H_2 overset(300^oC)-> 2NaH ; 2K + H_2 -> 2KH`
`2Na + Cl_2 -> 2NaC l; 2K + Cl_2 -> 2KCl`
`2Na + S -> Na_2S; 2K + S -> K_2S`
(ii) Li reacts, however directly with carbon and nitrogen to form carbides and nitrides.
`2Li + 2C -> LiCz; 6Li + 2N_2 -> 2 Li_3N`
(iii) The nitrides of these metals on reaction with water give `NH_3 + M_3N + 3H_2O -> 3MOH + NH_3`
(l) Reaction wlth acidic hydrogen : Alkali metals react with acids and other compounds containing aciclic hydrogen (i.e, II atom anached on F,O, N and triply bonded carbon atom, for example, `HF, H_2O, ROH, RNH_2, CHequivCH)` to liberate `H_2`.
`M +H_2O -> MOH +1/2 H_2 ; M + HX -> MX +1/2 H_2 `
`M + ROH -> ROH +1/2H_2 ; M +RNH_2 -> RNHNa 1/2H_2`
(m) Complex ion formation : A metal shows complex formation only when it possesses the following characteristics, (i) Small size (ii) High nuclear charge (iii) Presence of empty orbitals in order to accept electron pair ligand. Only Lithium in alkali metals due to small size forms a few complex ions Rest all alkali metals do not possess the tendency to form complex ion.
(a) Occurrence : Alkali metals are very reactive and thus found in combined state Some important ores of alkali metals are given ahead.
(i) Lithuinr : Triphylite, Petalite, lepidolite, Spodumene [`LiAl` `( SiO _3)_3`] Amblygonite [ `Li (Al F )PO _4`]
(ii) Sodium : Chile salt petre ( `NaNO_ 3`), Sodium chloride ( `NaCl `), Sodium sulphate (`Na _2 SO_ 4`),
Borax ( `Na_2 B_4O_ 7 10 H _2 O` ), Glauber salt ( `Na _2 SO_4*10H_ 2 O`)
(iii) Potassium : Sylime (`KCl` ), carnallite ( `KCl *MgCl_2 *6 H _2O` )and felspar (`K _2 O * Al_ 2 O_3 *6Si O_2`)
(iv) Rubidium : Lithuim ores Lepidolite, triphylite contains `0.7` to ` 3%` `Rb _2 O`
(v) Caesium : Lepidolite, Pollucite contains `0.2` to `7% Cs_2 O`
(b) Extraction of alkali metals : Alkali metals cannot be extracted by the usual methods for the extraction of metals due to following reasons.
(i) Alkali metals are strong reducing agents, hence cannot be extracted by reduction of their oxides or other compounds.
(ii) Being highly electropositive in nature, it is not possible to apply the method of displacing them from their salt solutions by any other element.
(iii) The aqueous solutions of their salts cannot be used for extraction by electrolytic method because hydrogen ion is discharged at cathode instead of an alkali metal ions as the discharge potentials of alkali metals are high. However, by using `Hg` as cathode, alkali metal can be deposited. The alkali metal readily combines with `Hg` to form an amalgam from which its recovery difficult. The only successful method, therefore, is the electrolysis of their fused salts, usually chlorides. Generally, another metal chloride is added to lower their fussion temperature.
Fused NaCl :
`NaCl overset(fusion)-> Na^+ +Cl^(-) `
Electrolysis of fused salt : Anode : ` 2Cl^(-) ->Cl_2 +2e`
cathode: `2Na^(+) +2 e-> 2 Na`
(c) Alloys Formation :
(i) The alkali metals form alloys among themselves as well as with other metals.
(ii) Alkali metals also get dissolved in mercury to form amalgam with evolution of heat and the amalgamation is highly exothermic.
(d) Formation of oxides and hydroxides :
(i) These are most reactive metals and have strong affinity for `O_2` quickly tranish in air due to the formation of a film of their oxides on the surface. These are, therefore, kept under kerosene or paraffin oil to protect them from air,
`M+O_2-> undersettext(oxide)(M_2O)-> undersettext(peroxide)(M_2O_2)`
(ii) When burnt air (`O_2`), lithium forms lithium oxide (`Li _2 O`) sodium forms sodium peroxide (`Na_2 O_2`) and other alkali metals form super oxide (`MO_2` i.e. `KO _2* RbO _2` or `CsO _2`)
`2Li+1/2 O_2-> Ki_2O; 2Na+O_2-> Na_2O_2; K+O_2-> KO_2`
The reactivity of alkali metals towards oxygen to form different oxides is due to strong positive field around each alkali metal cation. `Li ^+` being smallest, possesses strong positive field and rhus combines with small anion `O^(2-)` to form stable `Li_2O` compound.
The `Na^+` and `K^+` being relatively larger thus exert less strong positive field around them and thus reacts with larger oxygen anion i.e, `O_2^(2-)`to form stable oxides.
The monoxide, peroxides and superoxides have `O^2` and `O_2^(2-)`, `O_2^(1-)` ions respectively. The structures of each are
`[: undersettext(x x)overset(..)O:]^(-2)`, `[: underset(..)overset(..) O - underset(..) overset(..)O:]`, `[:overset(..)O...overset(..)O:]`
The `O_2^(-1)` ion has a three electron covalent bond and has one electron unpaired. It is therefore superoxides are paramagnetic and coloured `KO _2` is light yellow and paramagnetic substance.
(iii) The oxides of alkali metals and metal itself give strongly alkaline solution in water with evolution
of heat
`M+H_2O-> MOH+1/2 H_2 ; Delta=-ve`
`LiO_2 +H_2O ->2LiOH; Delta H=-ve`
`Na_2O_2+2H_2O->2NaOH+H_2O; Delta H=-ve`
`2KO_2+2H_2O->2KOH +H_2O+O_2; Delta H=-ve`
The peroxides and superoxides act as strong oxidising agents due to formation of `H_2O_2`
(iv) The reactivity of alkali metals towards air and water increases from `Li` to `Cs` that is why Jithjum decomposes `H _2O` very slowly at `25^oC` whereas `Na` does so vigorously, K reacts producing a flame and `Rb`, `Cs` do so explosively.
`M+H_2O->MOH+1/2 H_2`
(v) The basic character of oxides and hydroxides of alkali metals increases from `Li` to `Cs`. This is due to the increase in ionic character of alkali metal hydroxides down the group which leads to complete dissociation and leads to increase in concentration of `OH^-` ions.
(e) Hydrides :
(i)These metal combines `H` to give white crystalline ionic hydrides of the general of the formula `MH`.
(ii) The tendency to form their hydrides, basic character and stability decreases from `Li` to `Cs` since the electropositive character decreases from `Cs` to `Li`.
`2M + H_2 -> 2MH`; Reactivity towards `H_2` is `Cs < Rb < K < Na < Li`
(iii) The metal hydrides react with water to give `MOH` & `H_2`; `MH + H_2O -> MOH + H_2`
(iv) The ionic nature of hydrides increases from `Li` to `Cs` because of the fact that hydrogen is present in the these hydrides as `H^-` and the smaller cation will produce more polarisation of anion (according to Fajan rule) and will develop more covalent character.
(v) The electrolysis of fused hydrides give `H_2` at anode. `(NaH_(text(fused))text(Contains)) Na^(+) text(and) H^(-) ` i.e.,
At cathode: `Na^(+) + e -> Na;` At anode: `H^(-) -> 1/2H_2 + e`
(vi) Alkali metals also form hydrides like `NaBH_4,` `Li.A I H_4` which are good reducing agent.
(f) Carbonates and Bicarbonate :
(i) The carbonates `(M_2CO_3)` & bicarbonates `(MHCO_3)` are highly stable to heat, where `M` stands for alkali metals.
(ii) The stability of these salts increases with the increasing electropositive character from Li to Cs. It is therefore Liz `CO_3` decompose on heating, `Li_2CO_3 -> Li_2O + CO_2`
(iii) Bicarbonates are decomposed at relatively low temperature,
`2MHCO_3 overset(300^oC)-> M_2CO_3 +H_2O +CO_2`
(iv) Both carbonates and bicarbonates are soluble in water to give alkaline solution due to hydrolysis of carbonate ions or bicarbonate ions.
(g) Halides :
(i) Alkali metals combine directly with halogens to form ionic halide `M^(+) X^(-)`.
(ii) The ease with which the alkali metals form halides increases from `Li` to `Cs` due to increasing electropositive character from `Li` to `Cs`.
(iii) Lithium halides however have more covalent nature. Smaller is the cation, more is deformation of anion and thus more is covalent nature in compound. Also among lithium halides, lithium iodide has maximum covalent nature because of larger anion which is easily deformed by a cation (The Fajan's rule) Thus covalent character in lithium halides is, `LiI > LiBr > LiCl > LiF`
(iv) These are readily soluble in water. However, lithium fluoride is sparingly soluble. The low solubility of `LiF` is due to higher forces of attractions among smaller `Li^(+)` and smaller `F^(-)` ions (high lattice energy).
(v) Halides having ionic nature have high m.pt. and good conductor of current. The melting points of halides shows the order, `NaF > NaCl > NaBr > NaI`
(vi) Halides of potassium, rubidium and caesium have a property of combining with extra halogen atoms forming polyhalides.
`KI + I_2 -> KI_3`; In `KI_(3(aq))` the ions `K^(+)` and `I_3^(-)` are present.
(h) Solubility in liquid `NH_3` :
(i) These metals dissolve in liquid NH 3 to produce blue coloured solution, which conducts electricity to an appreciable degree.
(ii) With increasing concentration of ammonia, blue colour starts changing to that of metallic copper after which dissolution of alkali metals in `NH_3` ceases.
(iii) The metal atom is converted into ammoniated metal in i.e. `M^(+) (NH_3)` and the electron set free combines with `NH_3` molecule to produce ammonia solvated electron .
`Na +(x +y) -> undersettext(Ammoniated cation){NH_3[Na(NH_3)_x]^+} + undersettext(ammoniated electron){[e(NH_3)]^-}`
(iv) It is the ammoniated electron which is responsible for blue colour, paramagnetic nature and reducing power of alkali metals in ammonia solution. However, the increased conductance nature of these metals in ammonia is due to presence of ammoniated cation and ammonia solvated electron.
(v) The stability of metal-ammonia solution decreases from `Li` to `Cs`.
(vi) The blue solution on standing or on heating slowly liberates hydrogen, `2M + 2NH_3 -> 2MNH_2 + H_2` . Sodamide `(NaNH_2)` is a waxy solid, used in preparation of number of sodium compounds.
(i) Nitrates : Nitrates of alkali metals (`MNO_3`) are soluble in water and decompose on heating. `LiNO_3` decomposes to give `NO_2` and `O_2` and rest all give nitrites and oxygen.
`2MNO_3 -> 2MNO_2 + O_2` (except Li) ; `4 LiNO_3 -> 2Li_2O + 4NO_2 + O_2`
(j) Sulphates :
(i) Alkali metals' sulphate have the formula `M_2SO_4`.
(ii) Except `Li_2SO_4,` rest all are soluble water.
(iii) These sulphates on fusing v.rith carbon form sulphides, `M_2SO_4 + 4C -> M_2S + 4CO`
(iv) The sulphates of alkali metals (except Li) form double salts with the sulphate of the trivalent metals like Fe, AI, Cr etc. The double sulphates crystallize with large munber of water molecules as alum. e.g. `K_2SO_4 . Al_2 (SO_4)_3. 24 H_2O`
(k) Reaction with non-metals :
(i) These have high affinity for non-metals. Except carbon and nitrogen, they directly react with hydrogen, halogens, sulphur, phosphorus etc. to form corresponding compounds on heating.
`2Na + H_2 overset(300^oC)-> 2NaH ; 2K + H_2 -> 2KH`
`2Na + Cl_2 -> 2NaC l; 2K + Cl_2 -> 2KCl`
`2Na + S -> Na_2S; 2K + S -> K_2S`
(ii) Li reacts, however directly with carbon and nitrogen to form carbides and nitrides.
`2Li + 2C -> LiCz; 6Li + 2N_2 -> 2 Li_3N`
(iii) The nitrides of these metals on reaction with water give `NH_3 + M_3N + 3H_2O -> 3MOH + NH_3`
(l) Reaction wlth acidic hydrogen : Alkali metals react with acids and other compounds containing aciclic hydrogen (i.e, II atom anached on F,O, N and triply bonded carbon atom, for example, `HF, H_2O, ROH, RNH_2, CHequivCH)` to liberate `H_2`.
`M +H_2O -> MOH +1/2 H_2 ; M + HX -> MX +1/2 H_2 `
`M + ROH -> ROH +1/2H_2 ; M +RNH_2 -> RNHNa 1/2H_2`
(m) Complex ion formation : A metal shows complex formation only when it possesses the following characteristics, (i) Small size (ii) High nuclear charge (iii) Presence of empty orbitals in order to accept electron pair ligand. Only Lithium in alkali metals due to small size forms a few complex ions Rest all alkali metals do not possess the tendency to form complex ion.