Hydrogen Spectrum
Bohr's model for hydrogen atom is based on the following postulates:
i) The electron in the hydrogen atom can move around the nucleus in a circular path of fixed radius and energy. These paths are called orbits, stationary states or allowed energy states. These orbits are arranged concentrically around the nucleus.
ii) The energy of an electron in the orbit does not change with time. However, the electron will move from a lower stationary state to a higher stationary state when required amount of energy is absorbed by the electron or energy is emitted when electron moves from higher stationary state to lower stationary state . The energy change does not take place in a continuous manner.
iii) The frequency of radiation absorbed or emitted when transition occurs between two stationary states that differ in energy by ∆E, is given by :
`v=(∆E)/h = (E_2 - E_1)/h`
Where `E_1` and `E_2` are the energies of the lower and higher allowed energy states respectively. This expression is commonly known as Bohr's frequency rule.
iv) The angular momentum of an electron in a given stationary state can be expressed as in equation
`m_e vr = n.h/(2pi)` `n = 1,2,3.............`
Thus an electron can move only in those orbits for which its angular momentum is integral multiple of `h/(2π)` that is why only
certain fixed orbits are allowed. The details regarding the derivation of energies of the stationary states used by Bohr, are quite complicated and will be discussed in higher classes. However, according to Bohr's theory for hydrogen atom:
a) The stationary states for electron are numbered n = 1,2,3.......... These integral numbers are known as Principal quantum numbers.
b) The radii of the stationary states are expressed as :
`r_n = n^2 a_0`
where `a_0 = 52,9` pm. Thus the radius of the first stationary state, called the Bohr orbit, is 52.9 pm. Normally the electron
in the hydrogen atom is found in this orbit (that is n=1). As n increases the value of r will increase. In other words the electron will be present away from the nucleus.
c) The most important property associated with the electron, is the energy of its stationary state. It is given by the expression.
`E_n = -R_H(1/n^2)` `n=1,2,3.....`
where `R_H` is called Rydberg constant and its value is `2.18xx10^(-18) J`. The energy of the lowest state, also called as the ground state, is
`E_1 = -2.18 xx 10^(-18)`
The energy of the stationary state for n = 2, will be
`E_2 = -2.18xx10^(-18)(1/2^2) = -0.545xx10^(-18)`
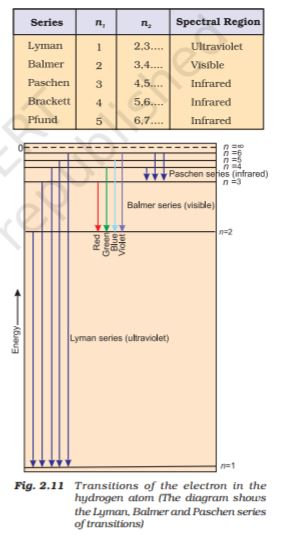
depicts the energies of different stationary states or energy levels of hydrogen
atom. This representation is called an energy level diagram.
When the electron is free from the influence of nucleus, the energy is taken as zero. The electron in this situation is associated with the stationary state of Principal Quantum number = n = ∞ and is called as ionized hydrogen atom. When the electron is attracted by the nucleus and is present in orbit n, the energy is emitted and its energy is lowered. That is the reason for the presence of negative sign in equation and depicts its stability relative to the reference state of zero energy and `n = ∞.`
d) Bohr's theory can also be applied to ) Bohr's theory can also be applied to the ions containing only one electron, similar to that present in hydrogen atom. For example, `He^+ Li^(2+) , Be^(3+)` and so on. The energies of the stationary states associated with these kinds of ions (also known as hydrogen like species) are given by the expression
`E_n = -2.18xx10^(-18)(Z^2 /n^2) J` and radii by the expression
`r_n = (52.9(n^2))/Z` pm
e) It is also possible to calculate the velocities of electrons moving in these orbits. Although the precise equation is not given here, qualitatively the magnitude of velocity of electron increases with increase of positive charge on the nucleus and decreases with increase of principal quantum number.
`text(Explanation of Line Spectrum of Hydrogen)`
Line spectrum observed in case of hydrogen atom can be explained quantitatively using Bohr's model. According to assumption 2, radiation (energy) is absorbed if the electron moves from the orbit of smaller Principal quantum number to the orbit of higher Principal quantum number, whereas the radiation (energy) is emitted if the electron moves from higher orbit to lower orbit. The energy gap between the two orbits is given by equation
`DeltaE = E_f - E_i`
Combining equations
`DeltaE = (-R_H/(n_f)^2) - (-R_H/(n_i)^2)`
(where `n_i` and `n_f` stand for initial orbit and final orbits)
`DeltaE = R_H(1/(n_i)^2 -1/(n_f)^2)`
The frequency (ν ) associated with the absorption and emission of the photon can be evaluated by using equation
`v = (DeltaE)/h = R_H/h (1/(n_i)^2 -1/(n_f)^2) = 3.29xx10^(15)(1/(n_i)^2 -1/(n_f)^2)`
`barv = v/c = R_H/(hc)(1/(n_i)^2 -1/(n_f)^2) = 1.09677 xx 10^7(1/(n_i)^2 -1/(n_f)^2) m^(-1)`
In case of absorption spectrum, `n_f > n_i` and the term in the parenthesis is positive and energy is absorbed. On the other hand in case of emission spectrum `n_i > n_f` , ∆ E is negative and energy is released.
The expression is similar to that used by Rydberg derived empirically using the experimental data available at that time. Further, each spectral line, whether in absorption or emission spectrum, can be associated to the particular transition in hydrogen atom. In case of large number of hydrogen atoms, different possible transitions can be observed and thus leading to large number of spectral lines. The brightness or intensity of spectral lines depends upon the number of photons of same wavelength or frequency absorbed or emitted.
i) The electron in the hydrogen atom can move around the nucleus in a circular path of fixed radius and energy. These paths are called orbits, stationary states or allowed energy states. These orbits are arranged concentrically around the nucleus.
ii) The energy of an electron in the orbit does not change with time. However, the electron will move from a lower stationary state to a higher stationary state when required amount of energy is absorbed by the electron or energy is emitted when electron moves from higher stationary state to lower stationary state . The energy change does not take place in a continuous manner.
iii) The frequency of radiation absorbed or emitted when transition occurs between two stationary states that differ in energy by ∆E, is given by :
`v=(∆E)/h = (E_2 - E_1)/h`
Where `E_1` and `E_2` are the energies of the lower and higher allowed energy states respectively. This expression is commonly known as Bohr's frequency rule.
iv) The angular momentum of an electron in a given stationary state can be expressed as in equation
`m_e vr = n.h/(2pi)` `n = 1,2,3.............`
Thus an electron can move only in those orbits for which its angular momentum is integral multiple of `h/(2π)` that is why only
certain fixed orbits are allowed. The details regarding the derivation of energies of the stationary states used by Bohr, are quite complicated and will be discussed in higher classes. However, according to Bohr's theory for hydrogen atom:
a) The stationary states for electron are numbered n = 1,2,3.......... These integral numbers are known as Principal quantum numbers.
b) The radii of the stationary states are expressed as :
`r_n = n^2 a_0`
where `a_0 = 52,9` pm. Thus the radius of the first stationary state, called the Bohr orbit, is 52.9 pm. Normally the electron
in the hydrogen atom is found in this orbit (that is n=1). As n increases the value of r will increase. In other words the electron will be present away from the nucleus.
c) The most important property associated with the electron, is the energy of its stationary state. It is given by the expression.
`E_n = -R_H(1/n^2)` `n=1,2,3.....`
where `R_H` is called Rydberg constant and its value is `2.18xx10^(-18) J`. The energy of the lowest state, also called as the ground state, is
`E_1 = -2.18 xx 10^(-18)`
The energy of the stationary state for n = 2, will be
`E_2 = -2.18xx10^(-18)(1/2^2) = -0.545xx10^(-18)`
depicts the energies of different stationary states or energy levels of hydrogen
atom. This representation is called an energy level diagram.
When the electron is free from the influence of nucleus, the energy is taken as zero. The electron in this situation is associated with the stationary state of Principal Quantum number = n = ∞ and is called as ionized hydrogen atom. When the electron is attracted by the nucleus and is present in orbit n, the energy is emitted and its energy is lowered. That is the reason for the presence of negative sign in equation and depicts its stability relative to the reference state of zero energy and `n = ∞.`
d) Bohr's theory can also be applied to ) Bohr's theory can also be applied to the ions containing only one electron, similar to that present in hydrogen atom. For example, `He^+ Li^(2+) , Be^(3+)` and so on. The energies of the stationary states associated with these kinds of ions (also known as hydrogen like species) are given by the expression
`E_n = -2.18xx10^(-18)(Z^2 /n^2) J` and radii by the expression
`r_n = (52.9(n^2))/Z` pm
e) It is also possible to calculate the velocities of electrons moving in these orbits. Although the precise equation is not given here, qualitatively the magnitude of velocity of electron increases with increase of positive charge on the nucleus and decreases with increase of principal quantum number.
`text(Explanation of Line Spectrum of Hydrogen)`
Line spectrum observed in case of hydrogen atom can be explained quantitatively using Bohr's model. According to assumption 2, radiation (energy) is absorbed if the electron moves from the orbit of smaller Principal quantum number to the orbit of higher Principal quantum number, whereas the radiation (energy) is emitted if the electron moves from higher orbit to lower orbit. The energy gap between the two orbits is given by equation
`DeltaE = E_f - E_i`
Combining equations
`DeltaE = (-R_H/(n_f)^2) - (-R_H/(n_i)^2)`
(where `n_i` and `n_f` stand for initial orbit and final orbits)
`DeltaE = R_H(1/(n_i)^2 -1/(n_f)^2)`
The frequency (ν ) associated with the absorption and emission of the photon can be evaluated by using equation
`v = (DeltaE)/h = R_H/h (1/(n_i)^2 -1/(n_f)^2) = 3.29xx10^(15)(1/(n_i)^2 -1/(n_f)^2)`
`barv = v/c = R_H/(hc)(1/(n_i)^2 -1/(n_f)^2) = 1.09677 xx 10^7(1/(n_i)^2 -1/(n_f)^2) m^(-1)`
In case of absorption spectrum, `n_f > n_i` and the term in the parenthesis is positive and energy is absorbed. On the other hand in case of emission spectrum `n_i > n_f` , ∆ E is negative and energy is released.
The expression is similar to that used by Rydberg derived empirically using the experimental data available at that time. Further, each spectral line, whether in absorption or emission spectrum, can be associated to the particular transition in hydrogen atom. In case of large number of hydrogen atoms, different possible transitions can be observed and thus leading to large number of spectral lines. The brightness or intensity of spectral lines depends upon the number of photons of same wavelength or frequency absorbed or emitted.