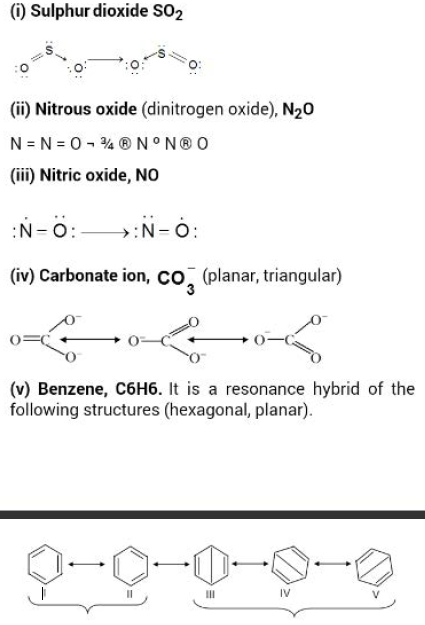
Resonance :
Carbon dioxide may be represented by Lewis dot formula as
` : overset( * *)O : : C : : overset( * *)O`
or `O = C = O ..............(1)`
The bond length of `C = O` is `1.22` `A^o`, but the actual measured value is `1.15` `A^o`. Further `CO_2` is quite stable and does not show the characteristic reactions of the carbonyl group, as shown by aldehydes and ketones. Without shifting, the relative positions of atoms of `CO_2` can be represented by two more Lewis formulae:
`O underset(->)= C -> O.....................(2) `
`O leftarrow C underset(leftarrow)= O................(3)`
In (2) and (3), the two bonds between `C` and `O` are different, one being a triple bond and the other a single bond. Both the `C-O` bonds in `CO_2` are identical. It is now obvious that none of these structures actually represents `CO_2`. To explain this difficulty the
concept of resonance was introduced, according to which `CO_2` cannot be accurately depicted by any Lewis formula. The actual structure of `CO_2` is a resonance hybrid of the three structures :
`O = C = O leftrightarrow O underset(->)= C -> O`
`leftrightarrow O leftarrow C underset(leftarrow)= O`
These different structures are called the canonical or contributing structures. The actual structure of `CO_2` is different from the canonical structures and although it is closely related to them, the actual structure cannot be represented on paper using the accepted symbols. All the molecules of `CO_2` have the same structure. Usually, a double-headed arrow `leftrightarrow` is used between the canonical structures.
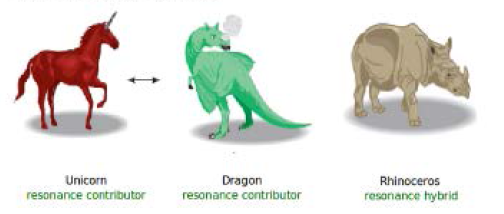
Imagine that you are trying to describe to a friend what a rhinoceros looks like. You might tell your friend that a rhinoceros looks like a cross between a unicorn and a dragon. See fig.
The unicorn and the dragon don't really exist, so they are like the resonance contributors. They are not in equilibrium. A rhinoceros does not jump back and forth between the two resonance contributors, looking like a unicorn one minute and a dragon the next. The rhinoceros is real, so it is like the resonance hybrid.
` : overset( * *)O : : C : : overset( * *)O`
or `O = C = O ..............(1)`
The bond length of `C = O` is `1.22` `A^o`, but the actual measured value is `1.15` `A^o`. Further `CO_2` is quite stable and does not show the characteristic reactions of the carbonyl group, as shown by aldehydes and ketones. Without shifting, the relative positions of atoms of `CO_2` can be represented by two more Lewis formulae:
`O underset(->)= C -> O.....................(2) `
`O leftarrow C underset(leftarrow)= O................(3)`
In (2) and (3), the two bonds between `C` and `O` are different, one being a triple bond and the other a single bond. Both the `C-O` bonds in `CO_2` are identical. It is now obvious that none of these structures actually represents `CO_2`. To explain this difficulty the
concept of resonance was introduced, according to which `CO_2` cannot be accurately depicted by any Lewis formula. The actual structure of `CO_2` is a resonance hybrid of the three structures :
`O = C = O leftrightarrow O underset(->)= C -> O`
`leftrightarrow O leftarrow C underset(leftarrow)= O`
These different structures are called the canonical or contributing structures. The actual structure of `CO_2` is different from the canonical structures and although it is closely related to them, the actual structure cannot be represented on paper using the accepted symbols. All the molecules of `CO_2` have the same structure. Usually, a double-headed arrow `leftrightarrow` is used between the canonical structures.
Imagine that you are trying to describe to a friend what a rhinoceros looks like. You might tell your friend that a rhinoceros looks like a cross between a unicorn and a dragon. See fig.
The unicorn and the dragon don't really exist, so they are like the resonance contributors. They are not in equilibrium. A rhinoceros does not jump back and forth between the two resonance contributors, looking like a unicorn one minute and a dragon the next. The rhinoceros is real, so it is like the resonance hybrid.