Dipole Moment :
The electronegativities of the two atoms which form the covalent bond are not the same. The atom having higher electronegativity will draw the bonded electron pair more towards itself resulting in a partial charge separation. The distribution of the electron cloud in the bond does not remain uniform and shifts towards the more eletronegative one. Such bonds are called polar covalent bonds. For example the bond formed between hydrogen and chlorine or between hydrogen and oxygen in water is of this type.
` overset(delta +)H - overset(delta -)Cl`
`overset(delta +)H - overset(delta -) O - overset(delta +)H`
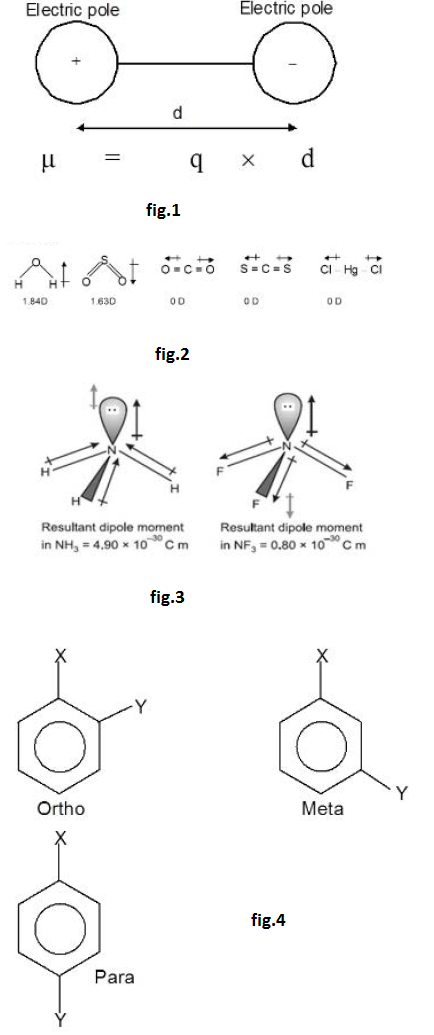
Molecules like `HCl`, `H_2O`, `NH_3` i.e. molecules of the type `H-X` having two polar ends (positive and negative) are known as polar molecules. The extent of polar character or the degree of polarity in a compound is given by it's dipole moment which is defined as the product of the net positive or negative charge and the distance of separation of the charges i.e. the bond length. The symbol of dipole moment is `mu`. It is vector quantity and is defined as the product of the magnitude of charge on any of the atom and the distance between the atoms. See fig.1.
The unit of dipole moment is Debye (`D`)
`1 D = 3.33xx10^(-30)` C-m `= 10^(- 18)` esu-cm
Dipole moment is indicated by an arrow having a symbol `( +rightarrow )` pointing towards the negative end. Dipole moment has both magnitude and direction and therefore it is a vector quantity.
Examples of Covalent compounds having diapole moment : See fig.2.
Let us study an interesting case of `NH_3` and `NF_3` molecule. Both the molecules have pyramidal shape with a lone pair of electrons on nitrogen atom. Although fluorine is more electronegative than nitrogen, the resultant dipole moment of `NH_3`(`4.90 xx10^(-30`) `C-m`) is greater than that of `NF_3` (`0.8 xx 10^(-30)` `C-m`). This is because. in case of `NH_3` the orbital dipole due to lone pair is in the same direction as the resultant dipole moment of the `N-H` bonds, whereas in `NF_3` the orbital dipole is in the direction opposite to the resultant dipole moment of the three `N-F` bonds. See fig.3
The dipole moments of the aromatic compounds present a very good illustration of dipole moment. We all know when a substituted benzene is treated with a reagent different products namely ortho, meta and para products are formed. The dipole moments of these products are different since the orientation of the groups are different at ortho, meta and para position. Let us take an example which will make it easily digestive for you. Suppose we have three isomers of o-nitrophenol, m-nitrophenol and p-nitrophenol. We have also the e.g. o-aminophenol, m-aminophenol and p-aminophenol. See fig.4.
`m_(text(ortho)) = sqrt(mu_1^2 +mu_2^2 + 2 mu_1 mu_2 cos 60^o)`
`m_(text(meta)) = sqrt(mu_1^2 +mu_2^2 +2 mu_1 mu_2 cos 120^o)`
`m_(text(para)) = sqrt(mu_1^2 +mu_2^2 +2 mu_1 mu_2 cos 180^o)`
` overset(delta +)H - overset(delta -)Cl`
`overset(delta +)H - overset(delta -) O - overset(delta +)H`
Molecules like `HCl`, `H_2O`, `NH_3` i.e. molecules of the type `H-X` having two polar ends (positive and negative) are known as polar molecules. The extent of polar character or the degree of polarity in a compound is given by it's dipole moment which is defined as the product of the net positive or negative charge and the distance of separation of the charges i.e. the bond length. The symbol of dipole moment is `mu`. It is vector quantity and is defined as the product of the magnitude of charge on any of the atom and the distance between the atoms. See fig.1.
The unit of dipole moment is Debye (`D`)
`1 D = 3.33xx10^(-30)` C-m `= 10^(- 18)` esu-cm
Dipole moment is indicated by an arrow having a symbol `( +rightarrow )` pointing towards the negative end. Dipole moment has both magnitude and direction and therefore it is a vector quantity.
Examples of Covalent compounds having diapole moment : See fig.2.
Let us study an interesting case of `NH_3` and `NF_3` molecule. Both the molecules have pyramidal shape with a lone pair of electrons on nitrogen atom. Although fluorine is more electronegative than nitrogen, the resultant dipole moment of `NH_3`(`4.90 xx10^(-30`) `C-m`) is greater than that of `NF_3` (`0.8 xx 10^(-30)` `C-m`). This is because. in case of `NH_3` the orbital dipole due to lone pair is in the same direction as the resultant dipole moment of the `N-H` bonds, whereas in `NF_3` the orbital dipole is in the direction opposite to the resultant dipole moment of the three `N-F` bonds. See fig.3
The dipole moments of the aromatic compounds present a very good illustration of dipole moment. We all know when a substituted benzene is treated with a reagent different products namely ortho, meta and para products are formed. The dipole moments of these products are different since the orientation of the groups are different at ortho, meta and para position. Let us take an example which will make it easily digestive for you. Suppose we have three isomers of o-nitrophenol, m-nitrophenol and p-nitrophenol. We have also the e.g. o-aminophenol, m-aminophenol and p-aminophenol. See fig.4.
`m_(text(ortho)) = sqrt(mu_1^2 +mu_2^2 + 2 mu_1 mu_2 cos 60^o)`
`m_(text(meta)) = sqrt(mu_1^2 +mu_2^2 +2 mu_1 mu_2 cos 120^o)`
`m_(text(para)) = sqrt(mu_1^2 +mu_2^2 +2 mu_1 mu_2 cos 180^o)`