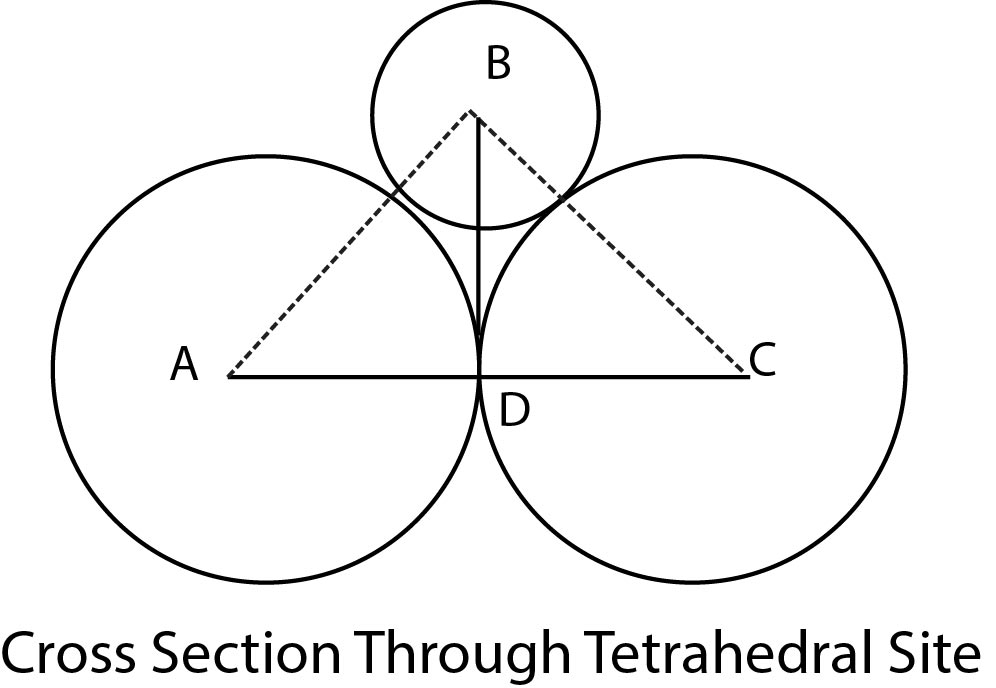
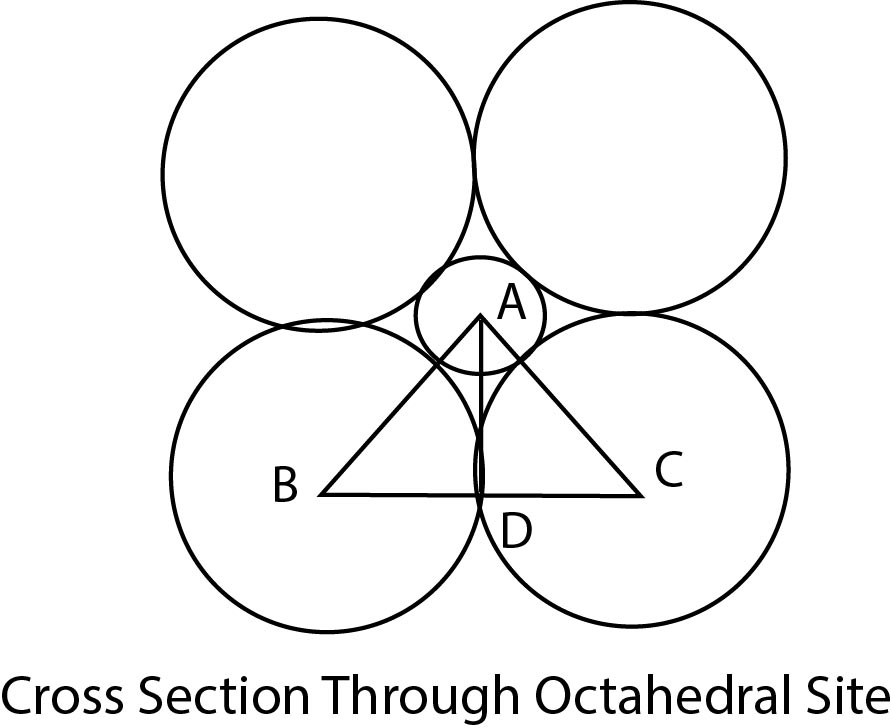
Co-ordination Number 3 :
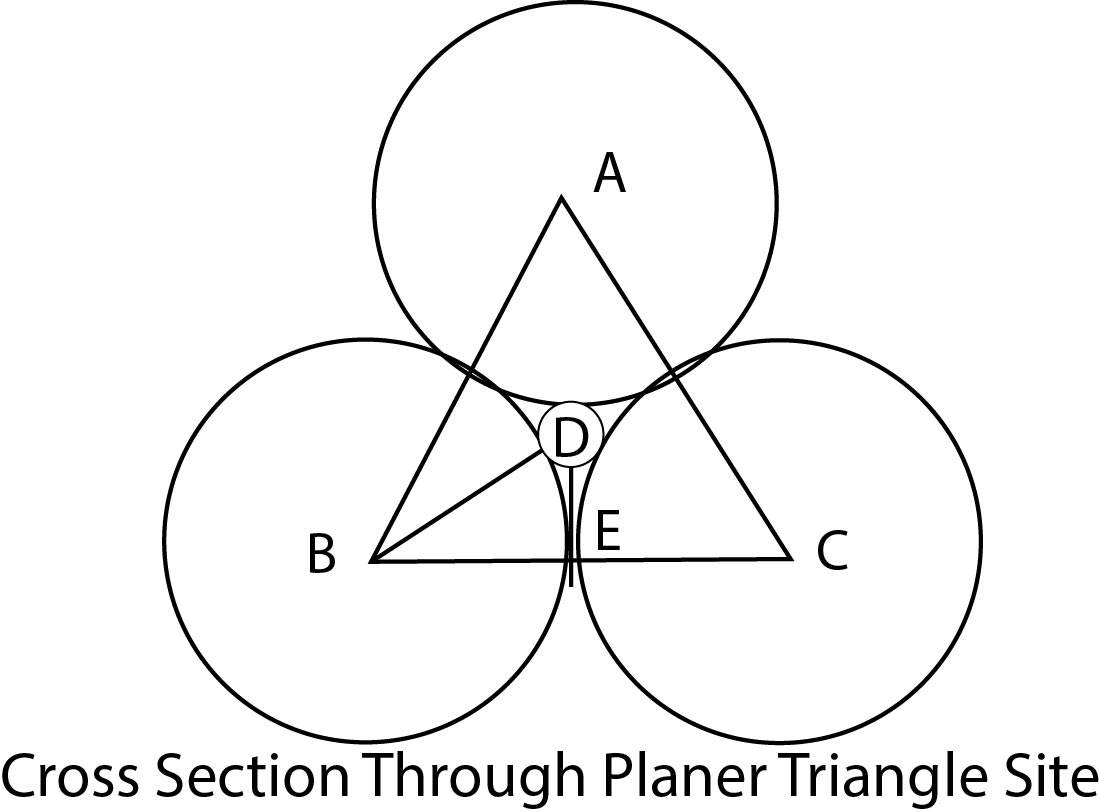
The adjacent fig. shows the smaller positive ion of radius `r^(+)` is in contact with three larger negative ions of radii `r^(-)`. It can be seen that `AB = BC = AC = 2r^(-)`, `BD = r^(-) + r^(+)`. Further, the angle ABC is `60�`, and the angle DBE is `30�`.
By trigonometry `Cos 30�` `= (BE // BD).`
`BD =` (`BE //cos 30�`), `r^(+) + r^(-) = r^(-) //cos 30� =` `(r^(-) // 0.866`) `= r^(-) xx 1. 155`, `r^(+) = (1 .155 r^(-)) -r^(-)`
`= 0.155 r^(-).`
Hence `(r^(+) //(r^(-)) = 0. 155.`
By trigonometry `Cos 30�` `= (BE // BD).`
`BD =` (`BE //cos 30�`), `r^(+) + r^(-) = r^(-) //cos 30� =` `(r^(-) // 0.866`) `= r^(-) xx 1. 155`, `r^(+) = (1 .155 r^(-)) -r^(-)`
`= 0.155 r^(-).`
Hence `(r^(+) //(r^(-)) = 0. 155.`